A team of scientists from the University of California, Los Angeles (UCLA) has achieved a significant milestone in scientific imaging. They have developed a groundbreaking improvement to a technology previously honored with a Nobel Prize. This achievement promises to enhance the capabilities of this technology and open up new avenues for research and discovery.
In 2017, some extraordinary scientists won a Nobel Prize for creating a fantastic technology called cryo-electron microscopy, or cryo-EM. It clearly lets them see tiny things, like the atoms in biological stuff.
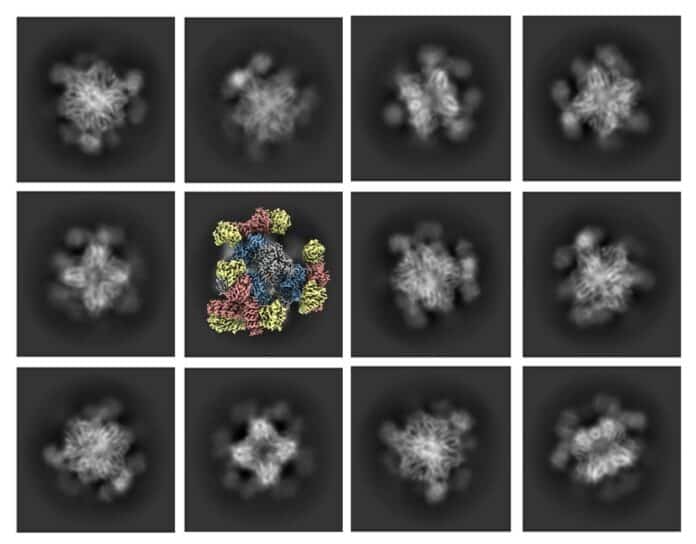
But there was a problem. Cryo-EM was great for big things but could have been better for small stuff, like little proteins. Now, scientists from UCLA have fixed that issue with some help from pharmaceutical experts. They made a tiny, cube-shaped structure 20 times smaller than a human hair. It’s like a scaffold with little tripod legs that hold the small proteins still when they’re being looked at.
They can later remove this scaffold from the image when they take pictures, leaving just the tiny protein in 3D. Small proteins are essential because they can help us make new medicines for challenging diseases. This new technique was tested on a protein that might be useful for treating cancer.
Scientists believe this will help them find unique spots on proteins they can target with medicines to help sick people improve.
Scientists use a special microscope to shoot electrons through frozen samples in cryo-EM. This makes thousands of pictures of the molecules in the sample, like proteins. These pictures show the molecules in the sample, like many 2D photos taken from different sides. Computers then put all these photos together to make a 3D picture. They figure out which part is the molecule and which is just the background, giving a precise 3D image.
But for really tiny proteins, they’re so small that it’s hard to tell how they’re sitting in the pictures. This means the images could be clearer.
In the past, scientists tried to solve this problem by sticking small molecules onto more considerable supports. However, these attempts didn’t work well because if the small molecules were too flexible, they stuck out in different ways, causing the pictures to be blurry.
Todd Yeates, a professor at UCLA, explained that “The blurriness happened because the computer couldn’t figure out the right way the molecules were facing.”
In the new study, the scientists made a scaffold with tripod-like parts that grab onto the proteins and keep them still. This helped create clearer pictures.
Roger Castells-Graells, a researcher at UCLA, mentioned that they tried different shapes for the scaffold before settling on the one with tripod-shaped parts.
The researchers initially tried a scaffold with one protrusion sticking out, but it didn’t work well. They then created a new scaffold with tripod-like protrusions that point toward each other in three groups, like tripods, to hold the protein firmly.
They tested this scaffold on a protein called KRAS, which is involved in many cancers. Pharmaceutical researchers are interested in KRAS because finding specific spots on the protein related to its cancer-causing abilities could help design drugs to treat cancer.
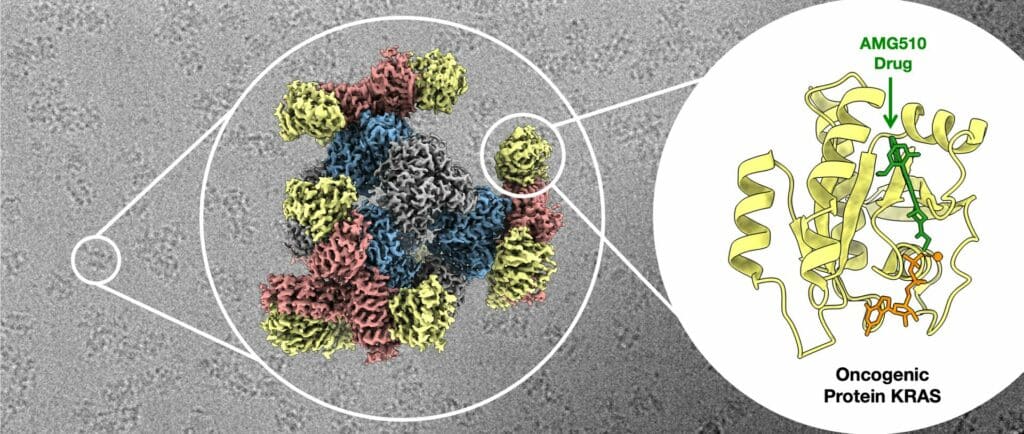
Using their new scaffold with cryo-EM, the UCLA-led team saw the atomic structure of KRAS when it was connected to a drug being studied for lung cancer treatment. This showed that their method can help understand how drugs interact with proteins like KRAS, potentially leading to better medicines.
Castells-Graells said, “The potential applications for the new advance don’t stop with cancer drugs. “Our modular scaffold can be assembled in any configuration to capture and hold all small protein molecules.”
The UCLA-led team’s essential improvement to cryo-EM technology represents a significant milestone in structural biology and scientific imaging. Their achievement in visualizing small therapeutic protein targets at 3 Å resolution is a testament to the power of innovation and collaboration in pushing the boundaries of scientific discovery. This breakthrough promises to revolutionize drug development and our understanding of complex biological systems, further solidifying Cryo-EM’s place as an invaluable tool in modern research.
Journal Reference:
- Roger Castells-Graells, Kyle Meador, et al., Cryo-EM structure determination of small therapeutic protein targets at 3 Å-resolution using a rigid imaging scaffold. Proceedings of the National Academy of Sciences. DOI: 10.1073/pnas.2305494120.