Reverse osmosis (RO) is the dominant desalination technology, mainly due to its high energy efficiency and low operating costs compared to other desalination technologies. RO is also a central component in advanced municipal wastewater reuse, a growing technology for alleviating water scarcity in water-stressed regions.
In a recent study, a group of scientists found that the conventional theory for reverse osmosis, which has been accepted for more than five decades, needs to be revised. The researchers present a competing theory in the process. These discoveries could result in more efficient usage of reverse osmosis and rectifying the record.
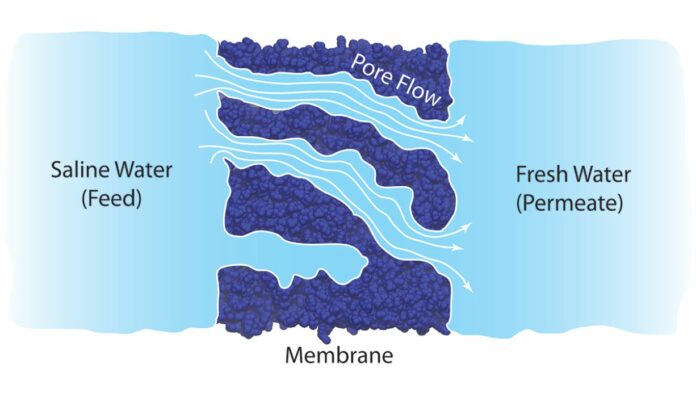
According to the theory of solution-diffusion, the water molecules dissolve and diffuse through the membrane by way of their concentration gradient — that is, the molecules move from areas of high concentration to wherever there are fewer molecules. However, some of its assumptions doesn’t make any sense. For example, the theory is partly based on the idea that pressure across the membrane is constant. There is always a pressure drop whenever water flows through any porous material.
The research team, which comprised experts from Texas Tech University and the University of Wisconsin-Madison, used a combination of computer simulations and tests to gain a deeper understanding of the physics at play. They specifically used molecular movement simulations, which showed that pressure variations within the membranes drive water transport.
They also demonstrate how water molecules move through the membrane’s network of holes in clusters. In contrast, the traditional hypothesis based on the solution-diffusion model contends that the membranes cause the water molecules to separate from one another. The scientists conducted tests that enabled them to watch the water move across membranes in addition to computer simulations. The findings demonstrated that the size of the membrane pores, the size of the water molecules, and the viscosity of the water all affect how water permeates the membrane. Additionally, this was at odds with the solution-diffusion model.
Prof. Menachem Elimelech, the Sterling Professor of Chemical and Environmental Engineering, said, “Overall, the simulations and experiments demonstrated that rather than being driven by the concentration of molecules, reverse osmosis is driven by pressure changes within the membrane.”
“Because previous measurements related to reverse osmosis were based on a faulty understanding of it on a molecular level, many attempts to advance the field have met a dead end. Having a more accurate theory to explain reverse osmosis could open the way toward developing more effective materials and techniques to improve the process.”
Journal Reference:
- Li Wang et al. Water transport in reverse osmosis membranes is governed by pore flow, not a solution-diffusion mechanism. Science Advances. DOI: 10.1126/sciadv.adf8488