Hyperactivity of the cyclin-dependent kinase 5 (Cdk5) enzyme is a significant pathological factor in neurodegeneration. Cdk5 is often controlled by its interaction with co-activators p35 or p39. By being broken down by calpain into the shortened activator p25, which binds to Cdk5 and causes extended activation and altered substrate selectivity, p35 causes Cdk5 hyperactivity. In mouse models, eliminating the calpain cleavage site in p35 prevents the generation of p25, but this genetic method does not offer a viable therapeutic approach.
By inhibiting Cdk5, MIT neuroscientists have discovered a technique to cure neurodegeneration and other signs of the illness.
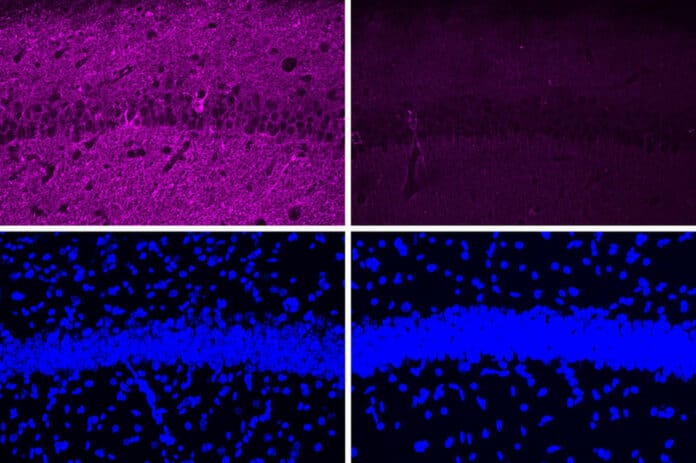
Neurodegeneration and DNA damage in the brain were significantly reduced in mice treated with a peptide that inhibits the hyperactive form of the CDK5 enzyme. Additionally, these mice showed improvements in their capacity to carry out tasks like learning a water maze.
In cells, CDK5 becomes more active when coupled to P25. Additionally, P25 enables CDK5 to phosphorylate molecules besides its typical targets, such as the Tau protein. The neurofibrillary tangles that are one of the defining characteristics of Alzheimer’s disease are created by hyperphosphorylated Tau proteins.
Previously, scientists have shown that transgenic mice engineered to express P25 develop severe neurodegeneration. In humans, P25 has been linked to several diseases.
The MIT team chose to use a peptide rather than a tiny molecule to target P25 in a new way. They created a peptide with a sequence that matches a region of CDK5 known as the T loop, a structure essential for CDK5 binding to P25. The complete peptide is just 12 amino acids long, slightly longer than the majority of currently available peptide medications, which range in length from five to ten amino acids.
The peptide therapy caused a moderate decrease in CDK5 activity, according to studies performed on neurons grown in lab dishes. Additionally, those studies revealed that the peptide does not affect other cyclin-dependent kinases or the typical CDK5-P35 complex.
The peptide had a variety of positive benefits when it was evaluated in a mouse model of Alzheimer’s disease that includes hyperactive CDK5, including a decrease in DNA damage, brain inflammation, and neuron loss. Comparing testing on cultured cells to mice experiments, these effects were considerably more obvious.
A different mouse model of Alzheimer’s disease that possesses a mutant variant of the Tau protein that results in neurofibrillary tangles responded dramatically better to the peptide treatment. Both the disorders caused by Tau and the loss of neurons were reduced in those mice after therapy. The researchers saw changes in behavior in addition to these impacts on the brain. Mice treated with the peptide outperformed mice treated with a control peptide (a scrambled version of the peptide used to suppress CDK5-P25) in a task that requires learning to navigate a water maze, which depends on spatial memory.
In those mouse studies, the researchers injected the peptide and found that it could cross the blood-brain barrier and reach neurons of the hippocampus and other parts of the brain.
The researchers also examined the modifications in gene expression that occur in mouse neurons after exposure to the peptide. One of the modifications they noticed was a rise in the expression of roughly 20 genes generally turned on by the MEF2 family of gene regulators. In prior research, Tsai’s team showed that MEF2 activation of these genes could give resilience to cognitive impairment in the brains of persons with Tau tangles. She hypothesizes that the peptide treatment may have a similar impact.
Stuart Lipton, a professor of neuroscience at Scripps Research, who was not involved in the study, said, “Further development of such peptide inhibitors toward a lead therapeutic candidate if proven to be selective for the target and relatively free of clinical side effects, may eventually lead to novel treatments for neurodegenerative disorders ranging from Alzheimer’s disease to Frontotemporal dementia to Parkinson’s disease.”
Journal Reference:
- Ping-CHeih Pao, Jinsoo Seo, et al. A Cdk5-derived peptide inhibits Cdk5/p25 activity and improves neurodegenerative phenotypes. PNAS. DOI: 10.1073/pnas.2217864120