The production of H2 at high turnover frequencies (TOFs) using earth-abundant molecular catalysts is an active area of research for replacing platinum as the dominant catalyst in hydrogen evolution.
The proposed mechanism includes reactive intermediates known as Ni-SIa, Ni-R, Ni-L, and Ni-C states, which regulate the proton and electron transfer steps. The Ni center is the redox-active site, switching between paramagnetic and diamagnetic states, whereas the Fe center remains divalent throughout catalysis.
However, the characterization and isolation of intermediates involved in mononuclear Ni electrocatalysts, which are reported to operate through a NiI/III cycle, still need to be discovered. Nickel-iron hydrogenase, an ancient biological enzyme, may be key in producing hydrogen for a renewable energy economy.
Chemists from the University of Illinois Urbana-Champaign designed a synthetic molecule miming the enzyme’s hydrogen gas-producing chemical reaction.
Industrial hydrogen is usually produced by electrolysis, which separates hydrogen gas molecules from oxygen atoms in water. However, many studies have shown that platinum’s expensiveness and limited availability make it unattractive as the world moves towards more environmentally friendly energy sources.
According to chemistry professor Liviu Mirica, who led the study with graduate student Sagnik Chakrabarti, Nature’s nickel-iron hydrogenase enzyme produces hydrogen using earth-abundant metals in its core.
Chakrabarti said, “The nickel at the core of the natural enzyme produces hydrogen by reducing protons in water. During the catalytic process, the nickel center goes through paramagnetic intermediates, meaning that the intermediates have an unpaired electron, making them extremely short-lived.”
Since more than ten years ago, synthetic chemists have created nickel compounds that generate hydrogen, but most function through non-paramagnetic intermediates.
Mirica from the University of Illinois Urbana-Champaign said, “Researchers are trying to mimic exactly what nature does because it is efficient, and maximizing efficiency is a key challenge to overcome when engineering energy sources. Being able to reproduce the paramagnetic intermediate steps that occur in the natural enzyme is what our group is trying to achieve to increase efficiency and mimic nature.”
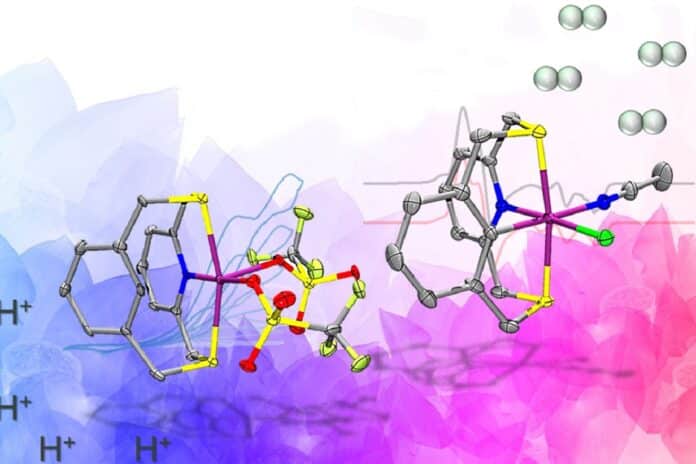
The team created a ligand, an organic compound that holds the nickel in place and supports the two relevant paramagnetic states that generate hydrogen. It contains electron-donating atoms like nitrogen and sulfur.
A carbon-hydrogen bond close to the nickel center, which is broken and reformed during catalysis, is the primary design feature that distinguishes this molecule from other catalysts. This was essential for maintaining the previously mentioned paramagnetic states.
Chakrabarti said, “One of the key takeaways from our work is that by using the specially designed ligand in the manner we did, we have successfully united ideas from two fields of inorganic chemistry – bioinorganic and organometallic chemistry – to make nickel complexes that behave similarly to the active site of one of nature’s most beautiful and complicated enzymes.”
According to the researchers, Several unusual enzymes with metal-carbon bonds in their active sites have recently been discovered. Such synthetic complex design principles could lead to new insights into how nature performs chemistry with small molecules like hydrogen.
This study was funded by the National Science Foundation.
Journal Reference:
- Chakrabarti, S., Sinha, etal. Characterization of paramagnetic states in an organometallic nickel hydrogen evolution electrocatalyst. Nature Communications. DOI: 10.1038/s41467-023-36609-7