Technologies for storing and transporting hydrogen bridge the gap between sustainable energy production and fuel use and, therefore, an essential component of a viable hydrogen economy. But traditional means of storage and transportation are expensive and susceptible to Contamination.
Hence, scientists are exploring alternative techniques that are reliable, low-cost, and simple.
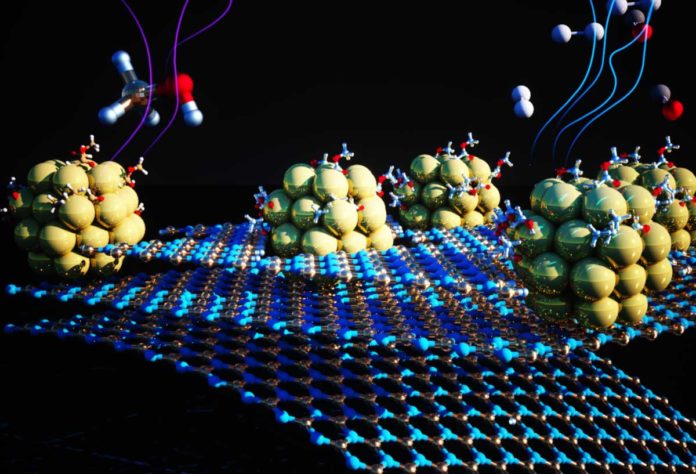
Scientists from Lawrence Berkeley National Laboratory (Berkeley Lab) have developed a material to optimize one of the limiting steps in extracting hydrogen from alcohols. The material that acts as a catalyst is made from tiny nickel-metal clusters anchored on a 2D substrate.
During experiments, scientists found that the catalyst could cleanly and efficiently accelerate the reaction that removes hydrogen atoms from a liquid chemical carrier.
Scientists developed the material using earth-abundant metals. The catalyst is expected to make hydrogen a viable energy source for a wide range of applications.
Jeff Urban, the Inorganic Nanostructures Facility director at the Molecular Foundry who led the work, said, “We present here not merely a catalyst with higher activity than other nickel catalysts that we tested, for an essential renewable energy fuel, but also a broader strategy toward using affordable metals in a broad range of reactions.”
Chemical compounds that act as catalysts like the one developed by Urban and his team are commonly used to increase the rate of a chemical reaction without the compound itself being consumed—they might hold a particular molecule in a stable position or serve as an intermediary that allows a critical step to be reliable to complete.
For the chemical reaction that produces hydrogen from liquid carriers, the most effective catalysts are made from precious metals. However, those catalysts are associated with high costs and low abundance and are susceptible to Contamination. Other less expensive catalysts, made from more common metals, tend to be less effective and less stable, limiting their activity and practical deployment into hydrogen production industries.
Postdoctoral research assistant Zhuolei Zhang and project scientist Ji Su, both at the Molecular Foundry and co-lead authors on the paper, designed and performed an experiment that combatted clumping by depositing 1.5-nanometer-diameter nickel clusters onto a 2D substrate made of boron and nitrogen engineered to host a grid of atomic-scale dimples. The nickel clusters became evenly dispersed and securely anchored in the dimples. Not only did this design prevent clumping, but its thermal and chemical properties greatly improved the catalyst’s overall performance by directly interacting with the nickel clusters.
Urban and his colleagues modified a strategy that focuses on tiny, uniform clusters of nickel-metal to improve these earth-abundant metal-based catalysts’ performance and stability. Tiny clusters are essential because they maximize the exposure of reactive surfaces in a given amount of material. But they also tend to clump together, which inhibits their reactivity.
The role of the underlying surface during the cluster formation and deposition stage has been critical and may provide clues to understanding their role in other processes.
The size of the catalyst is the reason behind its activity was among the best relative to others. Using models and computational methods, scientists uncovered the tiny metal clusters’ unique geometric and electronic structures.
Bare metal atoms, abundant on these tiny clusters, more readily attracted the liquid carrier than larger metal particles. These exposed atoms also eased the chemical reaction steps that strips hydrogen from the carrier while preventing the formation of contaminants that may clog the cluster’s surface.
Urban said, “Contamination can render possible non-precious metal catalysts unviable. Our platform here opens a new door to engineering those systems.”
In their catalyst, the scientists achieved the goal of creating a relatively inexpensive, readily available, and stable material that helps to strip hydrogen from liquid carriers for use as a fuel. This work came out of a DOE effort to develop hydrogen storage materials to meet the targets of EERE’s Hydrogen and Fuel Cell Technologies Office and to optimize the materials for future use in vehicles.
Journal Reference:
- Zhuolei Zhang et al. Enhanced and stabilized hydrogen production from methanol by ultrasmall Ni nanoclusters immobilized on defect-rich h-BN nanosheets. DOI: 10.1073/pnas.2015897117