One approach that shows promise for turning this industrial waste into useful chemicals is the electrochemical reduction of carbon dioxide (CO2). However, the catalyst must be near an electrode surface to carry out this reaction using a small-molecule catalyst. Customizing the attachment techniques for every situation has impeded efforts to bind molecular catalysts to electrodes.
An effective technique for converting carbon dioxide into carbon monoxide, a precursor chemical that may be used to create valuable molecules like ethanol and other fuels, has been created by chemical engineers at MIT.
When used extensively for industrial applications, this technology has the potential to mitigate climate change by lowering greenhouse gas emissions from sources like power plants.
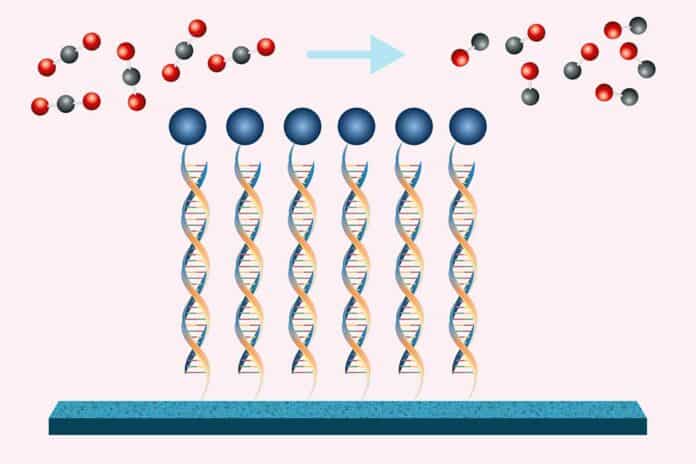
With the help of a catalyst attached to the electrode surface by DNA strands, the novel method uses energy to propel the chemical conversion. When the reaction’s constituents are kept near to one another by these DNA strands acting as Velcro, the reaction’s efficiency is much increased as opposed to when the constituents are spread out in solution.
Usually, carbon dioxide must first be converted into carbon monoxide to be processed into useful goods. Electrocatalysis is one way to accomplish this using electricity. However, this method is expensive because it frequently uses much energy.
Researchers have considered employing electrocatalysts to speed up the process and lower the required energy input to overcome this difficulty. For this reason, porphyrins—a class of molecules that contain metals like iron or cobalt—have been studied as catalysts. These molecules share structural similarities with oxygen-carrying heme molecules present in blood.
In an apparatus with an electrode that powers the reaction, carbon dioxide is dissolved in water to initiate an electrochemical reaction. In the solution, the catalysts are also distributed. Unfortunately, because the carbon dioxide and catalysts have to come into contact at the electrode surface, which doesn’t happen very often, this configuration is not particularly efficient.
Scientists investigated ways to affix the catalysts to the electrode surface to increase the reaction frequency and, consequently, improve the efficiency of the electrochemical conversion. They discovered that DNA might be the best choice in this regard.
Ariel Furst, the Paul M. Cook Career Development Assistant Professor of Chemical Engineering and the study’s senior author said, “DNA is relatively inexpensive, you can modify it chemically, and you can control the interaction between two strands by changing the sequences. It’s like a sequence-specific Velcro with very strong but reversible interactions you can control.”
The researchers used two chemical handles, one on the DNA and one on the electrode, to bind individual DNA strands to a carbon electrode. It is possible to permanently link these handles by joining them together. The porphyrin catalyst also has a corresponding DNA sequence connected to it. Like Velcro, the catalyst will bond reversibly to the DNA already linked to the electrode when added to the solution.
After the device is set up, the researchers give the electrode a potential known as a bias. With a tiny amount of hydrogen gas from the water, the catalyst uses this energy to change the carbon dioxide in the solution into carbon monoxide. Once the catalysts have become inactive, they can be eliminated from the surface by heating the mixture to disrupt the reversible connections separating the two DNA strands. New catalysts can then be used in their place.
By employing this technique, the researchers obtained an astounding 100% Faradaic efficiency, meaning that no energy was wasted and all electrical energy entered the system was directly used in the chemical reactions. By contrast, the Faradaic efficiency was only about 40 percent when the catalysts were not attached to DNA.
The carbon electrodes used in the study are significantly more affordable than traditional metal electrodes, which means this technique has great potential for industrial-scale applications. The catalysts are also reasonably priced because they don’t include precious metals and only a little amount is needed on the electrode surface.
The researchers aim to explore the production of other valuable products such as methanol and ethanol by substituting different catalysts. Helix Carbon, a company founded by Furst, is actively engaged in further developing this technology for potential commercialization.
Journal Reference:
- Gang Fan, Nathan Corbin, Minju Chung, Thomas M. Gill, Evan B. Moore, Amruta A. Karbelkar, and Ariel L. Furst*. Highly Efficient Carbon Dioxide Electroreduction via DNA-Directed Catalyst Immobilization. Journal of the American Chemical Society Au. DOI: 10.1021/jacsau.3c00823