Between 24 and 50 million Americans have autoimmune diseases, where the immune system attacks the body. Most affected are women, with ratios like 9 to 1 for lupus. Stanford Medicine scientists of Stanford University link this to a fundamental difference between biological females and males. Understanding this may help predict and prevent autoimmune disorders.
Howard Chang, MD, PhD, professor of dermatology and of genetics and a Howard Hughes Medical Institute investigator, said, “As a practicing physician, I see a lot of lupus and scleroderma patients, because those autoimmune disorders manifest in skin. The great majority of these patients are women.”
Chang, a cancer research professor, and Dou, an essential life scientist, lead a study about women having two X chromosomes. In contrast, men have one X and one Y. The findings, published in Cell, explore the impact of the “silence” of the second X chromosome in females.
The Y chromosome has few active genes; living without it is possible. Women lacking Y chromosomes are fine. Each Cell, female or male, needs at least one X chromosome with many active genes. Yet, having two X chromosomes risks excessive protein production. Nature’s workaround, X-chromosome inactivation, shuts down one X chromosome in each Cell during early development, ensuring balanced protein levels. The study led by Howard Chang reveals that this process can contribute to autoimmune disorders. However, other factors also play a role, affecting people of both gender.
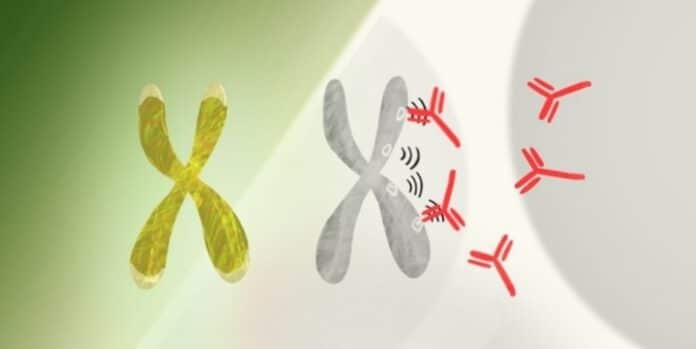
X-chromosome inactivation, controlled by Xist, ensures balance. Xist is an RNA molecule that acts as a messenger. Unlike traditional RNA, long noncoding RNA (lncRNA) like Xist doesn’t carry protein-making instructions but influences gene activity. In females, Xist, a type of lncRNA, coats one X chromosome, effectively silencing it. This process prevents the overproduction of proteins, maintaining cellular balance.
When Xist attaches to the extra X chromosome, it creates complex RNA, proteins, and DNA combinations. This mix can provoke a strong immune response, as Chang and his team discovered. A previous study identified nearly 100 proteins linked to Xist, many associated with autoimmune disorders.
This led to the question: Could the RNA-protein-DNA complexes formed during X-chromosome inactivation contribute to the higher rate of autoimmunity in women than men? This question prompted the new study.
To understand Xist’s impact, researchers inserted its gene into male lab mice. The modified gene could be turned on or off chemically, producing Xist only when needed. Even with the modified gene, the mice showed no apparent changes.
However, when activated, the Xist produced characteristic complexes with proteins linked to autoimmune disorders. This allowed scientists to compare the autoimmune susceptibility of bioengineered male mice producing Xist with normal males. This approach helped isolate Xist’s role, excluding influences like female hormones or silenced second X chromosomes in females.
Researchers induced a lupus-like autoimmune condition in susceptible mice. They compared its impact on males with activated Xist genes and normal males. In the susceptible strain, Xist-activated males developed lupus-like autoimmunity similar to females more than non-bioengineered males.
However, some females and Xist-activated males in this strain didn’t show autoimmunity, suggesting a combination of Xist activation and tissue-damaging stress is needed. Xist activation alone wasn’t enough in the autoimmune-resistant pressure, indicating that a specific genetic background is crucial for autoimmunity. These findings are critical to understanding susceptibility factors for autoimmune disorders.
Researchers aim to improve autoimmune screening by studying autoantibodies targeting Xist-related complexes. Blood samples from around 100 autoimmune patients revealed autoantibodies specific to certain disorders, suggesting their potential use in early detection. Some autoantibodies targeted common Xist-associated proteins across disorders, indicating they could serve as general markers for emerging autoimmune conditions before symptoms appear.
Chang said, “Every Cell in a woman’s body produces Xist. But we’ve used a male cell line as the reference standard for several decades. That male cell line produced no Xist and no Xist/protein/DNA complexes, nor have other cells used since for the test. So, all of a female patient’s anti-Xist-complex antibodies — a huge source of women’s autoimmune susceptibility — go unseen.”
This Stanford-led study significantly advances our understanding of why women face a higher risk of autoimmune diseases. By unraveling the complexities of X-chromosome inactivation and Xist’s role, the research opens avenues for more targeted diagnostic and therapeutic strategies, potentially improving outcomes for individuals at risk of autoimmune disorders.
Journal reference:
- Diana R. Dou, Yanding Zhao, et al., Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell. DOI: 10.1016/j.cell.2023.12.037.