Lithium-ion batteries are widely used for mobile power supply, but in some cases, their technology reaches its limits. As an alternative, lithium-metal batteries are gaining attention due to their higher energy density, lower weight, and longer lifespan. These rechargeable batteries utilize pure metallic lithium as one of their electrodes and are well-suited for various applications, including renewable energy storage, consumer electronics, and electric vehicles.
One of the main challenges that scientists face when trying to replace lithium-ion batteries with lithium-metal batteries is that – traditional electrolytes do not perform well in metal-based batteries. These electrolytes are essential as they allow electrical charges to move between a battery’s terminals and initiate the electrochemical reaction that converts the stored chemical energy into electric energy.
Now, a team of researchers, including engineers at Brown University and Idaho National Laboratory, suggest a potential solution that may lie in how soap works.
When soap is used, it forms micelles structures that trap and remove grease, dirt, and germs when flushed with water. The soap is a bridge between the water and what is being cleaned away.
Inspired by this mechanism, researchers created a new type of electrolyte called a localized high-concentration electrolyte that could help to overcome the obstacles inherent in metal-based batteries.
Localized high-concentration electrolytes are made by mixing high concentrations of salt in a solvent with another liquid called a diluent, which makes the electrolyte flow better so that the power of the battery can be maintained.
So far, in lab tests, this new type of electrolyte has shown promising results; however, how it works and why has never been fully understood. This limited the optimization and development of the electrolyte. The new study sheds light on this mystery.
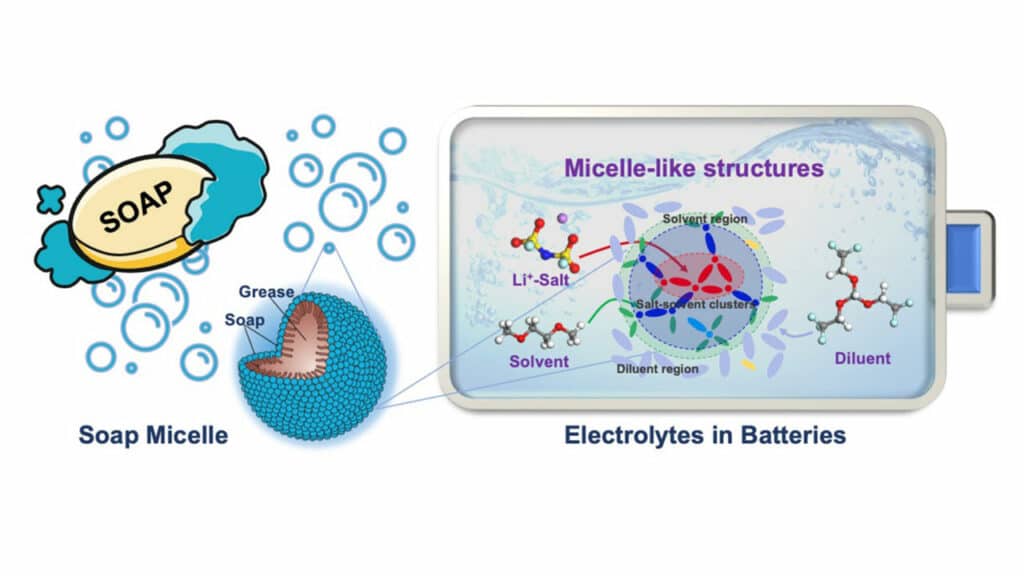
“The paper provides a unified theory as to why this electrolyte works better, and the key understanding of it came by finding that micelle-like structures form within this electrolyte – as they do with soap,” said Bin Li, a senior scientist at Oak Ridge National Laboratory who worked on the study. “Here we see that the role of the soap or surfactant is played by the solvent that binds both the diluent and the salt, wrapping itself around the higher concentration salt in the center of the micelle.”
Understanding this allowed researchers to break down the ratios and concentrations needed to bring about the optimal reactions for the batteries. This should help solve one of the main trouble points in engineering this electrolyte, which is finding the proper balance for the three ingredients. It will help engineers design better electrolytes that not only function but also work even more effectively.
Researchers at Idaho National Laboratory successfully put the theory into action and found that it helped to extend the life of lithium metal batteries.
“The concept of the micelle may be new for the electrolyte, but it’s actually very common in our daily life,” said Yue Qi, a professor at Brown’s School of Engineering, in a statement. “Now we have a theory, and we have guidelines to get interactions we want from the salt, the solvent, and the diluent in the electrolyte, and what concentration they have to be at and how you mix them.”
The team hopes that their work will inspire new designs for this promising electrolyte, but they also acknowledge that there are still challenges to overcome in creating high-density batteries. They are amused that the solution may have been hiding in something as common and simple as soap.
Journal reference:
- Corey M. Efaw, Qisheng Wu, Ningshengjie Gao, Yugang Zhang, Haoyu Zhu, Kevin Gering, Michael F. Hurley, Hui Xiong, Enyuan Hu, Xia Cao, Wu Xu, Ji-Guang Zhang, Eric J. Dufek, Jie Xiao, Xiao-Qing Yang, Jun Liu, Yue Qi, and Bin Li. Localized high-concentration electrolytes get more localized through micelle-like structures. Nature Materials, 2023; DOI: 10.1038/s41563-023-01700-3
