Obesity is responsible for various diseases, including cancer, as well as worse prognosis and survival. In many studies, scientists have studied obesity-related processes that cause tumor growth, like metabolic changes and chronic inflammation, yet the interplay between obesity and cancer has remained elusive.
To get a detailed understanding of this interplay, Harvard scientists conducted a new study on mice. They have uncovered that obesity allows cancer cells to outcompete tumor-killing immune cells in a battle for fuel.
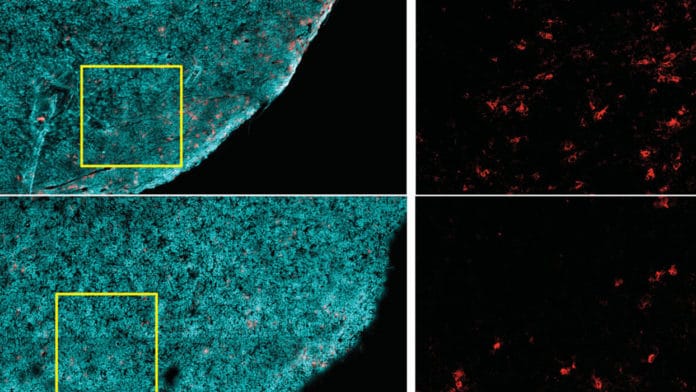
During the study, scientists fed mice with a high-fat diet. They then looked at different cell types and molecules inside and around tumors together, called the tumor microenvironment. They noticed that a high-fat diet reduces the numbers and antitumor activity of CD8+ T cells, a critical type of immune cell, inside tumors.
This happens because cancer cells reprogram their metabolism in response to increased fat availability to better gobble up energy-rich fat molecules, depriving T cells of fuel, and accelerating tumor growth.
Marcia Haigis, professor of cell biology in the Blavatnik Institute at HMS and co-senior author of the study, said, “Putting the same tumor in obese and nonobese settings reveals that cancer cells rewire their metabolism in response to a high-fat diet. This finding suggests that a therapy that would potentially work in one setting might not be as effective in another, which needs to be better understood given the obesity epidemic in our society.”
Blocking this fat-related metabolic reprogramming leads to reduced tumor volume in mice on high-fat diets.
Immunotherapies use CD8+ T cells to activate the immune system against cancer. The study suggests new strategies for improving such therapies.
Co-senior author Arlene Sharpe, the HMS George Fabyan Professor of Comparative Pathology, said, “Cancer immunotherapies are making an enormous impact on patients’ lives, but they do not benefit everyone. We now know there is a metabolic tug-of-war between T cells and tumor cells that changes with obesity.”
“Our study provides a roadmap to explore this interplay, which can help us to start thinking about cancer immunotherapies and combination therapies in new ways.”
Tumors grew rapidly when animals were fed with high-fat diets. Here is the point to note: this happens only in immunogenic cancer types, which can contain high numbers of immune cells.
The study also uncovered that the diet-related differences in tumor growth depended specifically on CD8+ T cells’ activity, immune cells that can target and kill cancer cells. Diet did not affect tumor growth rate if CD8+ T cells were eliminated experimentally in mice.
A high-fat diet eliminates the presence of CD8+ T cells in the tumor microenvironment. Those remaining in the tumor were less robust — they divided more slowly and had decreased activity markers. But when these cells were isolated and grown in a lab, they had regular activity, suggesting something in the tumor impaired these cells’ function.
Scientists also noticed that the tumor microenvironment consists of essential free fatty acids even if the rest of the body was enriched in fats, as expected in obesity.
These clues prompt scientists to conduct a comprehensive study of different cell types’ metabolic profiles in tumors under normal and high-fat diet conditions.
The analyses uncovered that fat availability changes cancer cell adoption. What’s more, they can reprogram their metabolism to increase fat uptake and utilization, while CD8+ T cells did not. This leads to depletion in specific fatty acids’ tumor microenvironment, leaving T cells starved for this essential fuel.
The study uses single-cell gene expression analyses, large-scale protein surveys, and high-resolution imaging to identify several diet-related changes to metabolic pathways of both cancer and immune cells in the tumor microenvironment.
When focused on PHD3, a protein in normal cells has been shown to act as a brake on excessive fat metabolism- scientists forced tumor cells to overexpress Ph.D. They found that this diminished a tumor’s ability to take up fat in obese mice. It also restored the availability of essential free fatty acids in the tumor microenvironment.
Scientists also found that low PHD3 expression was associated with immunologically “cold” tumors.
This association prompts scientists to conclude that tumor fat metabolism plays a role in human disease and that obesity reduces antitumor immunity in multiple cancer types.
Haigis said, “Our study provides a high-resolution metabolic atlas to mine for insights into obesity, tumor immunity, and the crosstalk and competition between immune and tumor cells. There are likely many other cell types involved and many more pathways to be explored.”
Journal Reference:
Journal Reference:
- Alison E. Ringel et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Antitumor Immunity. DOI: 10.1016/j.cell.2020.11.009