Hydrogen peroxide (formula H2O2) is a chemical compound that’s a combination of hydrogen and water. The clear liquid acts as a mild antiseptic and comes in various potencies depending on its purpose.
However, in remote villages in developing countries, where it could play an important role in health and sanitation, it can be hard to come by. MIT scientists now have found a solution to it.
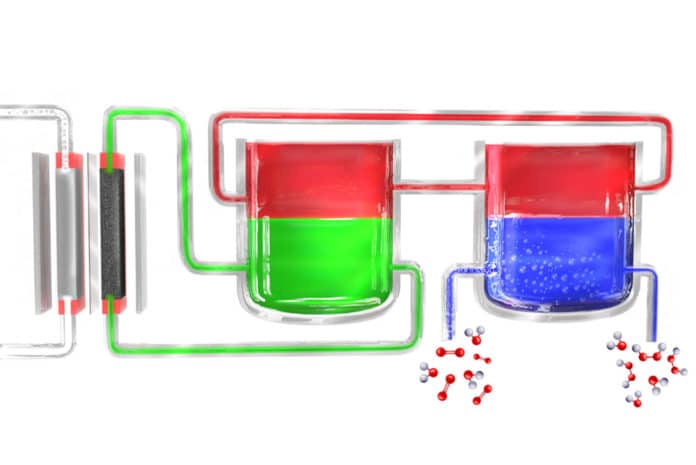
Scientists have developed a portable device that can make hydrogen peroxide continuously from just air, water, and electricity. Their new solution has made it easy to sterilize wounds, food preparation surfaces, and even water supplies.
The vast majority of the hydrogen peroxide created in the industrialized world is made in large chemical plants, where methane, or natural gas, is utilized to give a source of hydrogen, which is then reacted with oxygen in a catalyst process under high heat. This procedure is energy-intensive and not easily scalable, requiring large equipment and a consistent supply of methane, so it doesn’t fit smaller units or remote locations. However, other processes developed so far for potentially portable systems have key limitations.
In this new process, scientists used electricity to break down water into hydrogen and oxygen, and the hydrogen then reacts with a “carrier” molecule. This molecule — a compound called anthroquinone, in these initial experiments — is then introduced into a separate reaction chamber where it meets with oxygen taken from the outside air, and a pair of hydrogen atoms binds to an oxygen molecule (O2) to form the hydrogen peroxide. In the process, the carrier molecule is restored to its original state and returns to carry out the cycle all over again, so none of this material is consumed.
Yogesh Surendranath, a student at MIT said, “The process could address numerous challenges by making clean water, first-aid care for wounds, and sterile food preparation surfaces more available in places where they are presently scarce or unavailable.”
“Even at fairly low concentrations, you can use it to disinfect water of microbial contaminants and other pathogens. And, he adds, at higher concentrations, it can be used even to do what’s called advanced oxidation, where in combination with UV light it can be used to decontaminate water of even strong industrial wastes, for example from mining operations or hydraulic fracking.”
“In this initial proof-of-concept unit, the concentration of hydrogen peroxide produced is still low, but further engineering of the system should lead to being able to produce more concentrated output. One of the ways to do that is to just increase the concentration of the mediator, and fortunately, our mediator has already been used in flow batteries at really high concentrations, so we think there’s a route toward being able to increase those concentrations.”
“It’s kind of an amazing process because you take abundant things, water, air, and electricity, that you can source locally, and you use it to make this important chemical that you can use to actually clean up the environment and for sanitation and water quality.”
Shannon Stahl, a professor of chemistry at the University of Wisconsin said, “The ability to create a hydrogen peroxide solution in water without electrolytes, salt, base, etc., all of which are intrinsic to other electrochemical processes, is noteworthy. Access to salt-free aqueous solutions of H2O2 has broad implications for practical applications.”
Stahl says that “This work represents an innovative application of ‘mediated electrolysis.’ Mediated electrochemistry provides a means to merge conventional chemical processes with electrochemistry, and this is a particularly compelling demonstration of this concept. … There are many potential applications of this concept.”
Journal Reference:
- Alexander T. Murray et al. Electrosynthesis of Hydrogen Peroxide by Phase-Transfer Catalysis. DOI: 10.1016/j.joule.2019.09.019