Correctly identifying the malignancy of human tumors is crucial for selecting effective and safe treatment strategies. Bioimaging technologies that rely on luminescent molecules are commonly employed to pinpoint and differentiate active tumor cells.
A collaborative research effort, spearheaded by Professor Yasuchika Hasegawa and Professor Shinya Tanaka from the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) at Hokkaido University, has resulted in the development of a non-destructive Cancer Grade Probing System (GPS).
This system is designed to assess the malignancy grade of model glioma tumor cells using a water-soluble, luminescent europium complex. The innovative approach has the potential to pave the way for non-invasive tests to determine tumor malignancy in patients.
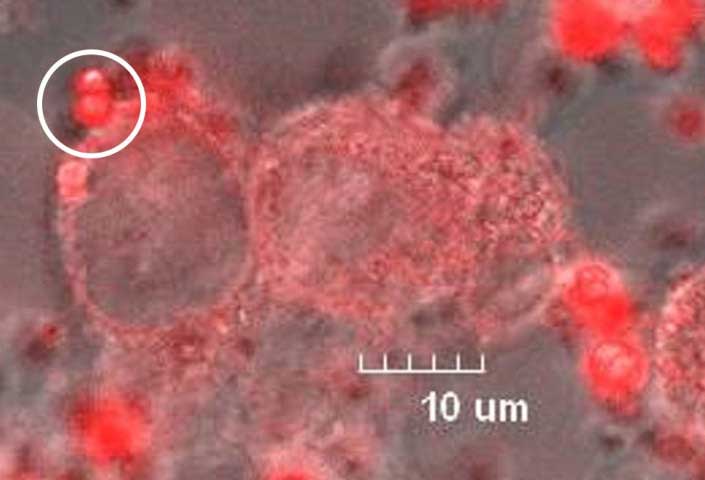
In their study, the research team assessed tumor malignancy by introducing the luminescent europium complex to model cells that mimic glioma, a prevalent type of tumor constituting 26.3% of brain cancers. They experimented with three distinct model cells representing different grades of malignancy. To evaluate changes, the researchers measured alterations in the lifetime of the characteristic red-light emission from the europium complex.
Their findings revealed that within the initial three hours after introducing the europium complex, more significant changes in the lifetime of the light emission occurred in the cells with higher malignancy.
Hasegawa said, “Visualization of cancer cells using luminescent complexes has previously been reported, but our hypothesis was that the photophysical signals sent by such complexes in cancer cells might reflect internal information from the cancer cells.”
To obtain the observed results, the researchers first modified the europium complex to enhance its water solubility and stability in the presence of amino acids in the cell culture medium. When introduced into the cell culture medium, the europium complex initially forms aggregates with itself.
However, these aggregates break down into single molecules upon interacting with model tumor cells. The cells rapidly absorb these individual molecules, triggering structural changes in the europium complex. These changes in the complex’s structure result in alterations to the lifetime of its red-light emission.
The observed differences in emission lifetimes were attributed to the distinct tumor activity and growth processes associated with different malignancy grades. These variations could lead to other structural changes in the europium complex at various time scales.
The researchers anticipate that employing this method could enable continuous detection of tumor activity, offering doctors essential information when deciding on appropriate treatment strategies. This non-destructive cancer-grade probing system has the potential to provide real-time insights into the dynamics of tumor behavior, aiding in the development of personalized and effective treatment approaches.
Journal Reference:
- Mengfei Wang, Masaya Kono, Yusaku Yamaguchi, Jahidul Islam, Sunao Shoji, Yuichi Kitagawa, Koji Fushimi, Sora Watanabe, Go Matsuba, Akihisa Yamamoto, Motomu Tanaka, Masumi Tsuda, Shinya Tanaka, and Yasuchika Hasegawa. Structure-changeable luminescent Eu(III) complex as a human cancer grade probing system for brain tumor diagnosis. Scientific Reports. January 22, 2024. DOI: 10.1038/s41598-023-50138-9