The MIT researchers are developing fluorescent polymer gels that change color when shaken, heated, unveiled to acid, or otherwise disorganized. This new material can become effective sensors for identifying changes in structures, fluids, or the environment.
The researchers combine a broadly used polymer with a metal that glows and a chemical for creating the gels. The chemicals that are used can wrap the two together. By mixing into a solvent, the metal and binder quickly assemble by themselves, grab the polymer molecules, and pull them compositely to form a gel. The researchers can control the physical properties of the gel by using different metals. Similarly, researchers can also change the color of light it emits. While testing, the gels diffused a color-coded reply to different external stimuli. Later it restored to their repeated state and color.
Muscles performing:
Natural organisms show some unusual behaviors. For example, the mussel creates strong fibers. This fiber permits it to join tightly to boats, rocks, and other underwater surfaces. But these fibers are also changeable. Taking out on an attached mussel, the stiff fibers become stretchy. After that by leaving behind and the fibers are returned to their original stiff state, “self-repairing” any damage that’s occurred. In comparison, man-made materials are typically not very active, and when they break, the damage is permanent.
Niels Holten-Andersen, assistant professor in the Department of Materials Science and Engineering (MSE), has long been interested in mussel fibers and the metal coordination complex. This structure is made up of a single metal ion with various chemically bound arms, or “ligands,” shining outside. The ligands are made by natural carbon-containing molecules and can join with other molecules. It enables the complex to supply as a crosslink that ties materials simultaneously. The metal coordination complex plays a vital role in many biological systems with this ability, including the human body, where it assembles enzyme-controlled reactions and binds oxygen to hemoglobin in the blood.
Niels said, “I am always been impressed by the way that nature assembles materials, putting together proteins, sugars, and fatty acids in creative ways to form complex vital structures. We can’t copy nature’s materials. For example, it’s difficult to synthesize proteins in the lab. But we can see how nature develops its materials and why they work the way they do. We can then try to act the way nature has done it but using simple, cheap developing blocks that we know how to make.”
Polymer developing blocks seemed like a good bet for Niels. He said that “he knows developing simple, cheap, green polymers in large quantities.” Before four years, he decided to make polymer gels bound simultaneously by metal coordination complexes developed on transition metals. Transition metals are a family of elements he had commonly seen in biological settings. The primary outcome was as expected. The polymer molecules, metals, and ligands quickly assembled themselves into gels. The gels’ mechanical properties and emitted colors depended on the transition metal used.
Adding fluorescence
Inspiring by that outcome, Niels decided to try the lanthanides, a different family of metals. Similar to transition metals, lanthanides are usually mentioned as rare-earth elements. This metal supplies a host of interesting and complicated behaviors. But they have one additional interesting characteristic: They glow. Shine an ultraviolet light on a lanthanide, and it becomes excited and emits light at a characteristic wavelength.
Holten-Andersen said, “By using the lanthanides, we could manage the properties of our gels. But now we have a light emission that would return any changes in those properties. With those two features nearly connects, any time the physical properties were disturbed. Because of changing temperature of the nearby air or the pH of the surrounding water, the color emitted would change.”
This type of polymer gel could inform its state and supply as an excellent sensor. For example, it could be used as a covering that monitors the structural integrity of pipes, cables, and other underwater structures complicated to seaward oil and gas and wind farm operations.
Testing in liquids
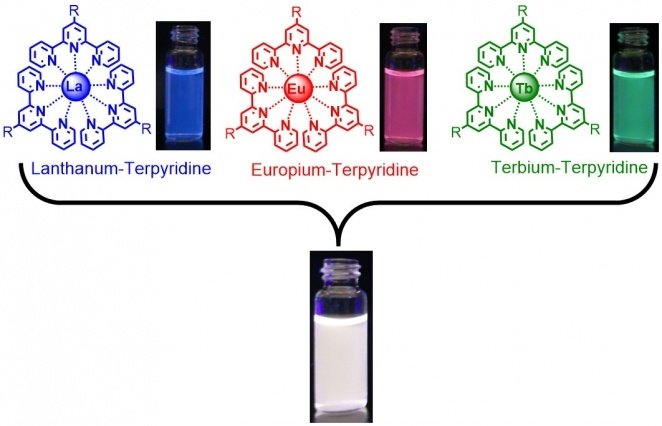
Before starting to work with polymers, Holten-Andersen wanted to confirm that, as others had shown, adding lanthanide ions and ligands in a solvent would generate light-emitting fluids. According to that, he and his team in the Laboratory combined terpyridine. Terpyridine is a commonly available ligand material, with selected lanthanides in a solvent. As the images in the slide show above describe, the mixtures generate liquids that glow under ultraviolet light. This light comes out with the characteristic colors of the three lanthanides. They are blue for lanthanum, red for europium, and green for the terbium.
This outcome shows that the complexes formed as expected. But a mixture emitting pure white light would make a far better sensor. It is easy to see the white light turn little green than it is to see the green light become less green. Through this, the researchers found that producing a white-light-emitting fluid was simple. However, white light is a combination of many colors. They just needed to mix together their blue, red, and green light-emitting fluids. As shown in Figure 1 in the slideshow above, putting together equal parts of the three colored fluids produced a glowing white liquid.
The researchers next unveiled their white-light-emitting fluid to a series of external stimuli to see if they were getting a color-coded response and they got. For example, when they lightly heated the fluid at room temperature to 55 degrees Celsius, the emitted light continuously changed color. When they cooled it, the white light got back. The ligand and metal ions had come separately when they were heated and then reassembled when they were allowed to cool. The fluid also proved responsive to various changes in ph.
So scientists found that this simple blue-red-green approach to making a white-light-emitting system causes materials to react to a variety of stimuli. Through this response, it comes color changes. The fluids might supply good sensors for identifying chemical changes within a liquid or observing velocity gradients in fluid flow experiments. Through imitation differences, inflows are determined indirectly.
Adding the polymer
In the next series of tests, the researchers combined their lanthanide ions and terpyridine ligands into commonly used polyethylene glycol or PEG. At the time of the experiment, the polymer molecules combined with ligands were independently floating in a solvent. The scientist then inserted in one of their lanthanide metals, and after some light shaking, the mixture changed from a fluid to a glowing gel. The metal ions and ligands had assembled themselves by joining the polymers together.
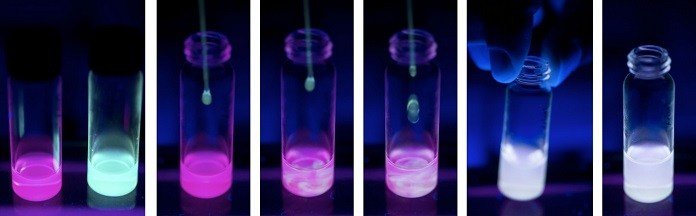
Again, scientists found that gels based on different lanthanides emitted different colors, and combining them in various ratios generates fluctuation in color. The lead image above shows a series of gels made using europium and terbium. The sample at the far left is all europium, therefore red; the one at the far right is all terbium, therefore blue-green; and those in between are made with various ratios of the two. Bright white luminescence is visible in the second sample from the right when the mixing ratio of terbium to europium is 96 to 4. The samples describe the simplicity of designing “metallogels” with a full spectrum of colors.
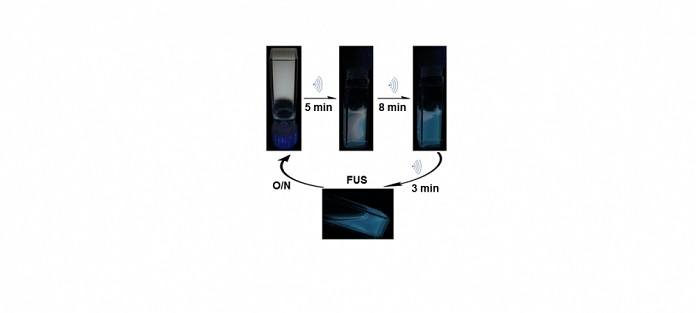
Like the fluids, the gels proved to be responsive identifiers of changes in temperature and pH. But the most dramatic response came when the gels were disrupted by exposure to high-frequency sound waves. Figure 2 above shows changes in the white-light-emitting gel during immersion in an ultrasonic bath. After 5 minutes, the gel is partially broken down into a fluid in the sample taken. The gel that remains shows its white luminescence, while the fluid gives off blue light. The now-unbound ligands emit it. After 11 minutes of continuous shaking, the sample changes from gel to the fluid are complete, and the blue light of the Ligands is controlled.
One more time, the white-light gel reassembles. “When we let the blue fluid rest overnight, the polymers found each other again, and it turned back into a gel and made white light. That was very exciting for us because it really shows in principle that as a proof-of-concept, our approach works under these conditions. We can make a material that emits white light, reports its own failure, and then recovers. So it’s a self-reporting material that’s also self-healing,” said Niels.
Looking ahead
Holten-Andersen and his team are now finding applications of their materials as coverings that can sense structural failure as well as pH and temperature changes. This is an ability that will be most important in many energy and environmental systems. Current work focuses on coatings for underwater cables used to deliver electric power from seaward wind turbines to shore.
The researchers are also planning more basic studies. There is a huge interest in making materials that can change in reactance to various outside stimuli and then repair by themselves and return to their original state. Such kind self-healing materials will decrease the need to manufacture replacements for them over time.
“Although, Knowing how to demonstrate self-healing materials, needs knowing how those materials fail and repair in the field, and that is difficult to study. I hope their new materials may help. The chemical bonds in the metal-coordinate crosslink have a special ability to break and then re-form and to announce that activity with changes in light emission. Guided by those light changes plus high-resolution imagery, we are able to get new insights into when, where, and how the material breaks and then comes back together,” said Holten-Andersen.
He then said, “We’re just scratching the surface in understanding nature. Given technology is a just example of we now have to look at it. For example, we far from investigating all the metal coordination complexes that nature uses. They could occur in other natural materials with special properties — perhaps in spider silk, which is tough, elastic, resilient, and one of the strongest materials known. It’s hard to see these metals. They appear in tiny concentrations and a single molecule at a time. But I think metal coordination complexes are much more prevalent in nature’s materials than we are currently aware of. And coordination complexes are just one among many tricks that nature has developed over millions of years to help organisms deal with challenging environmental conditions.”
Image Gallery