Mitochondria are the powerhouses of the cell. They break down molecules and manufacture new ones to generate the fuel necessary for life. Mitochondria depend on a surge of proteins to manage this vitality generation. Almost every one of their proteins is produced in the encompassing gel-like cytoplasm and must be foreign made into the mitochondria to keep the powerhouse running.
MIT scientists have identified what really happens when a traffic jam of proteins at the surface of the mitochondria prevents proper import. This is the first mechanism identified that surveils mitochondrial protein import, and aides mitochondria when they can’t get the proteins they require.
Scientists described that how interaction takes place between mitochondria and a cell to signal a problem. They also report how the cell responds to protect the mitochondria. According to them, this pathway called mitoCPR recognizes import setbacks and jam mitochondrial work amidst such pressure.
Angelika Amon, the Kathleen and Curtis Marble Professor of Cancer Research in the MIT Department of Biology said, “Responses to mitochondrial stress have been established before, but this one specifically targets the surface of the mitochondria, clearing out the misfolded proteins that are stuck in the pores.”
Mitochondria likely started as free elements long prior, before being immersed by having cells. They, in the end, surrendered control and moved the greater part of their imperative qualities to an alternate organelle, the core, where whatever is left of the cell’s hereditary diagram is put away.
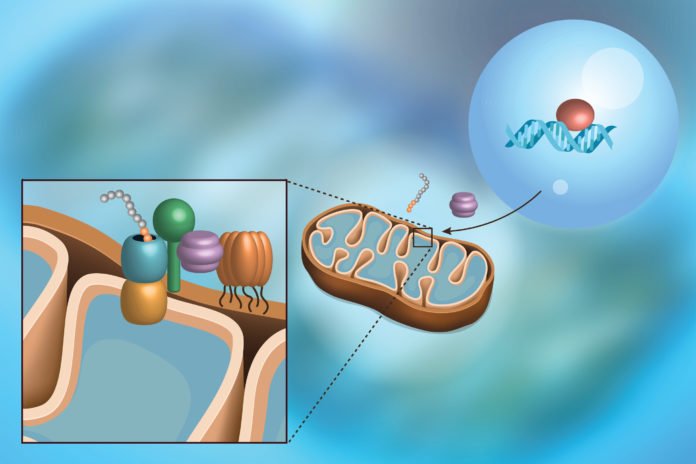
The protein items from these qualities are at last made in the cytoplasm outside the core, and after that guided to the mitochondria. These “forerunner” proteins contain an uncommon sub-atomic postal district that aides them through the channels at the surface of the mitochondria to their separate homes.
The proteins must be unfurled and carefully strung through the thin directs keeping in mind the end goal to enter the mitochondria. This makes a dubious circumstance; if the request is too high, or the proteins are collapsed when they shouldn’t be, a bottleneck frames that none might pass. This can just happen when the mitochondria grow to make a greater amount of themselves, or in infections like deafness-dystonia disorder and Huntington’s. genes.
Scientists identified mechanisms to evict proteins that are perched on the surface of the mitochondria and sends them for debasement. They also identified two other pathways in yeast that also respond to accumulated mitochondrial proteins. Those proteins refuse from the cytoplasm around the mitochondria, as opposed to expelling the proteins gathering on the mitochondria themselves.
Weidberg said, “We knew about various responses to mitochondrial stress, but no one had described a response to protein import defects that specifically protected the mitochondria, and that’s exactly what mitoCPR does. We wanted to know how the cell reacts to these problems, so we set out to overload the import machinery, causing many proteins to rush into the mitochondrion at the same time and clog the pores, triggering a cellular response.”
Vlad Denic, professor of molecular and cellular biology at Harvard University said, “What makes our cells absolutely dependent on mitochondria is one of those million-dollar questions in cell biology. This study reveals an interesting flip-side to that question: When you make mitochondrial life artificially tough, are they programmed to say ‘help us’ so the host cell comes to their rescue? The possible ramifications of such work in terms of human development and disease could be very impressive.”
Roughly two decades ago, scientists determined that mitoCPR is some kind of mechanism against mitochondrial dysfunction. Now, scientists finally characterized it, given it a name, and identified its precise function: to help mitochondrial protein import.
Scientists found that the protein that starts mitoCPR — the interpretation factor Pdr3 — ties to DNA inside the core, prompting the statement of a quality known as CIS1. The resultant Cis1 protein ties to the channel at the surface of the mitochondrion, and enrolls yet another protein, the AAA+ adenosine triphosphatase Msp1, to help clear unimported proteins from the mitochondrial surface and intercede their corruption. In spite of the fact that the MDR reaction pathway contrasts from that of mitoCPR, both depend on Pdr3 enactment. Truth be told, mitoCPR requires it.
Amon said, “Whether the two pathways interact with one another is a very interesting question. The mitochondria make a lot of biosynthetic molecules, and blocking that function by messing with protein import could lead to the accumulation of intermediate metabolites. These can be toxic to the cell, so you could imagine that activating the MDR response might pump out harmful intermediates.”
“It’s also yet to be determined whether an analogous pathway exists in more complex organisms, although there is some evidence that the mitochondria do communicate with the nucleus in other eukaryotes besides yeast.”
Amon said, “This was just such a classic study. There were no sophisticated high-throughput methodologies, just traditional, simple molecular biology and cell biology assays with a few microscopes. It’s almost like something you’d see out of the 1980s. But that just goes to show — to this day — that’s how many discoveries are made.”
The study appears in the journal Science.