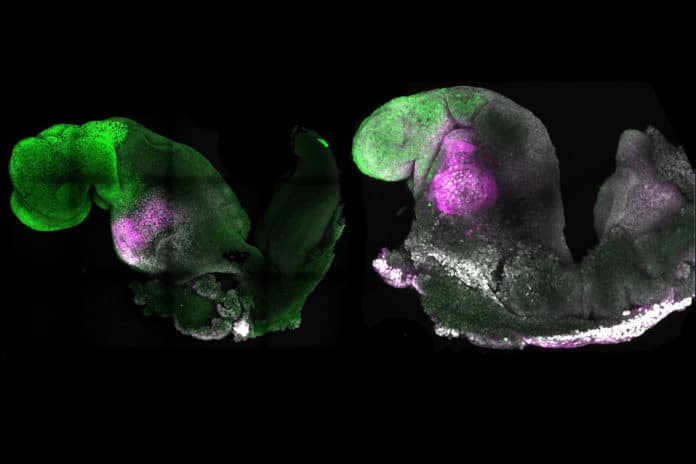
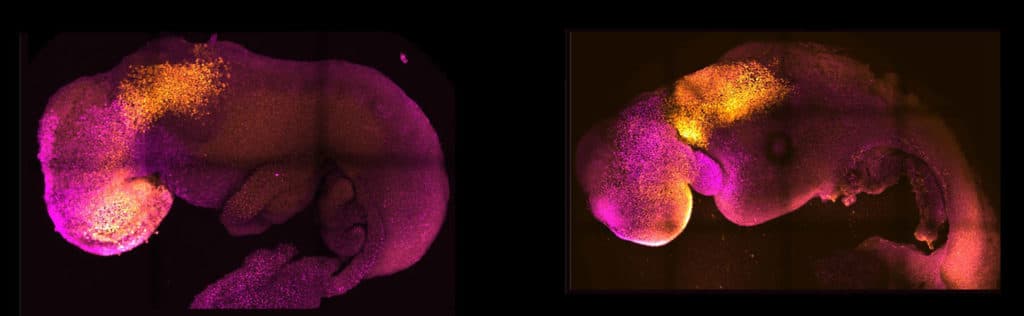
Scientists from the University of Cambridge and Caltech have successfully created synthetic mouse embryos- from embryonic stem cells- to form a brain, a beating heart, and the foundations of all the other organs of the body, a new avenue for recreating the first stages of life.
Instead of using eggs or sperm, scientists used stem cells, the body’s master cells, which can develop into almost any cell type in the body. They did this by mimicking natural processes in the lab- by directing the three types of stem cells present in early mammalian development to the stage where they begin interacting. They activated a specific set of gene expressions to create a unique environment for their (stem cells) interactions.
The stem cells self-organized into structures that progressed through the various developmental stages until the synthetic embryos had beating hearts and the foundations for a brain, as well as the yolk sac where the embryo develops and receives nutrients in its early weeks.
This is the most advanced stage of development in a stem cell-derived model to date.
Scientists noted, “Our embryo model displays head-folds with defined forebrain and midbrain regions and develops a beating heart-like structure, a trunk comprising a neural tube and somites, a tail bud containing neuromesodermal progenitors, a gut tube, and primordial germ cells. This complete embryo model develops within an extra-embryonic yolk sac that initiates blood island development.”
“A major advance in this study is the ability to generate the entire brain, particularly the anterior region, which has been a “holy grail” in the development of synthetic embryos.”
Magdalena Zernicka-Goetz, Bren Professor of Biology and Biological Engineering at Caltech, said, “This opens new possibilities to study the mechanisms of neurodevelopment in an experimental model. We demonstrate the proof of this principle in the paper by knocking out a gene already known to be essential for the formation of the neural tube, precursor of the nervous system, and for brain and eye development. In the absence of this gene, the synthetic embryos show exactly the known defects in brain development as in an animal carrying this mutation. This means we can begin applying this approach to the many genes with unknown function in brain development.”
“Our mouse embryo model not only develops a brain but also a beating heart, all the components that go on to make up the body. It’s just unbelievable that we’ve gotten this far. This has been the dream of our community for years and the major focus of our work for a decade, and finally, we’ve done it.”
Three different stem cell types begin to form within the first week of fertilization; one of these will eventually become the bodily tissues, while the other two will help the embryo’s growth. The placenta, which connects the fetus to the mother and supplies oxygen and nutrition, will be formed from one of these two types: extraembryonic stem cells. The other will develop into the yolk sac, where the embryo grows and gets its early nutrition.
Many pregnancies fail at the point when the three types of stem cells begin to send mechanical and chemical signals to each other, which tell the embryo how to develop properly.
To understand why some pregnancies are unsuccessful while others result in success, Zernicka-team Goetz’s has been researching these early phases of pregnancy for the past ten years.
Zernicka-Goetz said, “The stem cell embryo model is important because it gives us accessibility to the developing structure at a stage normally hidden from us due to the implantation of the tiny embryo into the mother’s womb. This accessibility allows us to manipulate genes to understand their developmental roles in a model experimental system.”
Scientists put together cultured stem cells representing each of the three tissue types to direct the formation of their synthetic embryos. They then allowed these cells to grow and communicate with one another in proportions, eventually leading to their self-assembly into an embryo.
The extraembryonic cells guide the embryo’s growth by mechanically sending chemical signals to the embryonic cells or through touch.
Zernicka-Goetz said, “This period of human life is so mysterious, so to be able to see how it happens in a dish—to have access to these individual stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening—is quite special. We looked at the dialogue that has to happen between the different types of stem cells at that time—we’ve shown how it occurs and how it can go wrong.”
While this research is being conducted on mouse models, scientists plan to develop an analogous model for human embryo development to understand the mechanisms behind crucial processes that would be otherwise impossible to study in actual embryos.
Zernicka-Goetz says, “If these methods are shown to be successful with human stem cells in the future, they could also be used to guide the development of synthetic organs for patients awaiting transplants. There are so many people around the world who wait for years for organ transplants.”
“What makes our work so exciting is that the knowledge coming out of it could be used to grow correct synthetic human organs to save lives that are currently lost. It should also be possible to affect and heal adult organs by using our knowledge of how they are made.”
Journal Reference:
- Amadei, G., Handford, C.E., Qiu, C. et al. Synthetic embryos complete gastrulation to neurulation and organogenesis. Nature (2022). DOI: 10.1038/s41586-022-05246-3