A new study from Scott B. Hansen’s lab in Jupiter, Florida, discovered how physical pressure on cells can reduce pain signals. Excessive Cholesterol in cell membranes can affect this process. The Herbert Wertheim UF Scripps Institute study shows that cell membrane fats send electrical pulses into cells after pressure. This shows how pain signals travel from injury sites to the brain and highlights how too much Cholesterol in cell membranes could disrupt pain control.
Hansen, an associate professor of molecular medicine at The Wertheim UF Scripps Institute, said, “Excess Cholesterol is a feature in many diseases and disorders, including diabetes and diseases of aging. This could be one explanation for why we see more chronic pain in these groups.”
The study further supports the phenomenon that the fatty molecules in cell membranes need structure to work correctly. Hansen mentioned that researchers initially believed only proteins had functional designs.
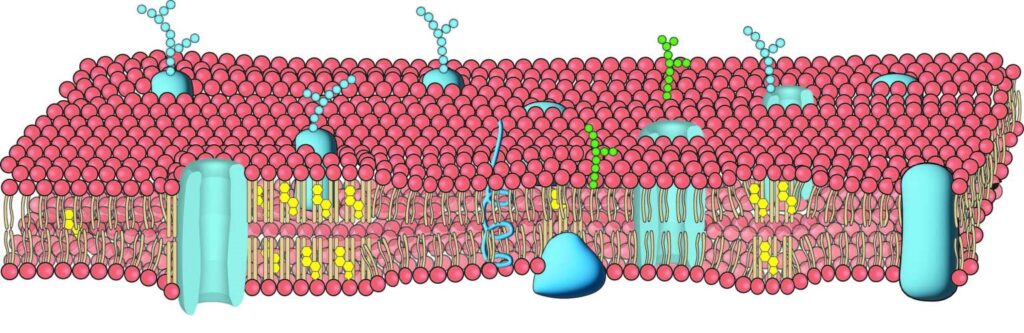
However, lipids can also have this function. Cell membranes are not just fatty bags; they are complex structures containing sensors, pores, channels, receptors, and cholesterol clumps arranged by fat molecules. Advanced microscopes and new technologies are studying these details.
Hansen explained that “cell membranes have two types of fats: fluid like olive oil, and the other contains Cholesterol, forming tiny, rigid clumps resembling lard. It was unknown that these fats might affect pain signaling.”
For pain to be felt, the injury must first be sensed and then converted into a signal that travels quickly through the body to be understood by the brain. Lipid structure seems to feel the force and convert it into a password, which can activate the body’s pain-relieving responses, reducing pain severity as long as there’s no interference.
Researchers previously knew about a force-sensing enzyme called PLD2 and its role in activating a pain-relief potassium channel called TREK-1. However, how the membrane could activate PLD2 and TREK-1 needed to be proven. PLD2 couldn’t sense tension, the usual way mechanosensory is activated, and membrane lipids weren’t considered due to limited understanding and technical challenges.
Hansen mentioned that “studying cholesterol-containing lipid clumps, known as lipid rafts, has been difficult due to their small size, which regular microscopes can’t see.”
Using a super-resolution microscope, Hansen and his team observed changes in fat molecules in various cell types when pressure or stretching occurred, affecting the cell’s ability to relieve pain temporarily. Studies in mice and fruit flies supported these findings.
The research prompts more questions and opportunities for study. Many proteins interact with these lipid structures, including those involved in Alzheimer’s disease and inflammation. Understanding how inflammation affects membrane cholesterol structure, especially in brain cells, could help explain the link between pain and inflammation.
Hansen emphasized the need for new non-opioid pain treatments for chronic pain sufferers. Understanding the factors influencing pain thresholds is crucial for developing such therapies.
Journal reference:
- E Nicholas Petersen, Mahmud Arif Pavel, et al., Mechanical activation of TWIK-related potassium channel by nanoscopic movement and rapid second messenger signaling. eLife. DOI:10.7554/eLife.89465.3.