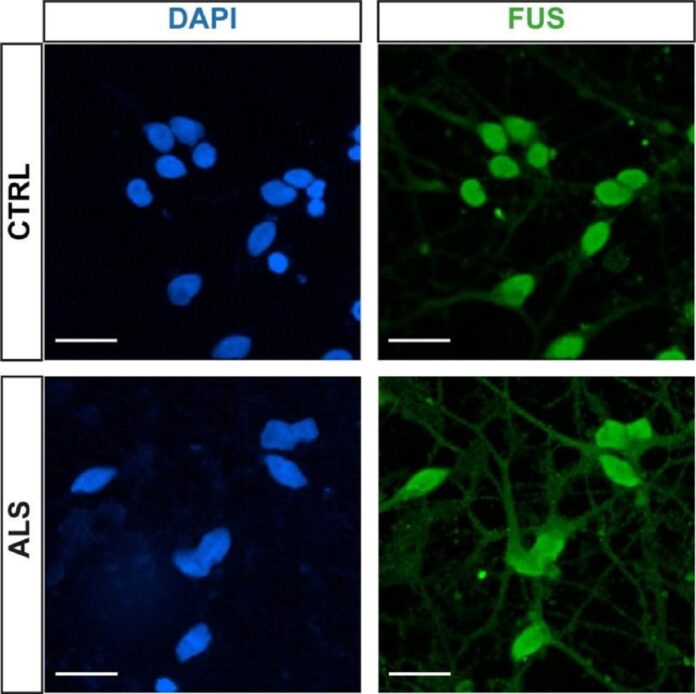
According to Francis Crick Institute and University College London researchers, hundreds of proteins and mRNA molecules are displaced in nerve cells affected by motor neuron disease (MND), known as amyotrophic lateral sclerosis (ALS).
ALS is a rapidly growing and deadly disease that causes paralysis by damaging motor neurons, with few therapy options. Until now, scientists had known that a few proteins, namely TDP-43, were located in unexpected places in ALS nerve cells.
However, new research published today in Neuron reveals that the problem is far more widespread. This ‘localization’ affects more proteins than previously anticipated, particularly RNA-binding ones. Mislocalization also affects mRNAs, which carry instructions to build proteins from DNA in the nucleus.
This mislocalization impacts far more proteins than previously anticipated, including those involved in RNA binding and mRNAs, which are molecules that convey instructions to produce proteins from DNA in the nucleus.
The researchers created motor neurons with ALS-causing mutations in the TARDBP and VCP genes using stem cells from patients. They separated the cell’s two main compartments (nucleus and cytoplasm) and examined the mRNA and protein within each. They discovered that hundreds of mRNAs and proteins were mislocated in ALS cells compared to healthy cells. Proteins and mRNAs moved from the cell’s nucleus (the ‘control center’) to the cytoplasm (the ‘body’), indicating potential transport difficulties.
The researchers also discovered that misplaced mRNAs and proteins interacted more with one another than those in the correct location. They believe that as mRNAs and proteins mislocalize, they drag each other along, causing a domino effect.
Oliver Ziff, the clinician-scientist at the Crick and University College London Hospitals NHS Foundation Trust (UCLH), said: “We were surprised to see the extent of the mislocalization, particularly for mRNAs, as this hasn’t been reported before. The goal now is to find where this problem starts, and there are many intriguing possibilities – one being a breakdown in the transport between the nucleus and cytoplasm. This study was an exceptional team effort. I’m immensely grateful to my colleagues, particularly co-first authors Drs Jasmine Harley, Yiran Wang, and Jacob Neeves”.
Surprisingly, a medicine called ML240, which inhibits the action of the VCP enzyme, improved protein and mRNA mislocalization. By inhibiting this enzyme, researchers improved cell function by lowering DNA damage.
This study offers a paradigm shift in our understanding of what causes ALS; it involves the aberrant movement of hundreds of proteins and mRNAs rather than just a handful.
The researchers will now look into protein and mRNA placement in various ALS genetic backgrounds, and there is still a long way to go before VCP inhibitors can be utilized clinically. ML240 has yet to be tested in animals, and potential chemical modifications may be required to ensure that it accesses nerve cells without producing negative effects.
Journal Reference:
- Jasmine Harley, Oliver J. Ziff, et al. Nucleocytoplasmic mRNA redistribution accompanies RNA binding protein mislocalization in ALS motor neurons and is restored by VCP ATPase inhibition. Neuron. DOI: 10.1016/j.neuron.2023.06.019