Researchers at the University of Illinois Urbana-Champaign and collaborators at the University of Wisconsin-Madison have developed a new antifungal molecule by modifying the structure of the well-known drug Amphotericin B. This new molecule retains its effectiveness against fungal infections while eliminating Amphotericin B’s toxicity to patients, especially their kidneys. The findings were reported in the journal Nature.
Research leader Dr. Martin D. Burke, an Illinois professor of chemistry, a professor in the Carle Illinois College of Medicine, and a medical doctor, said, “Fungal infections are a public health crisis that only worsens. And they have the potential, unfortunately, of breaking out and having an exponential impact, kind of like COVID-19 did. So let’s take one of the powerful tools that nature developed to combat fungi and turn it into a powerful ally.”
“This work demonstrates that, by going deep into the fundamental science, you can take a billion-year head start from nature and turn it into something that hopefully is going to have a big impact on human health,” Burke added.
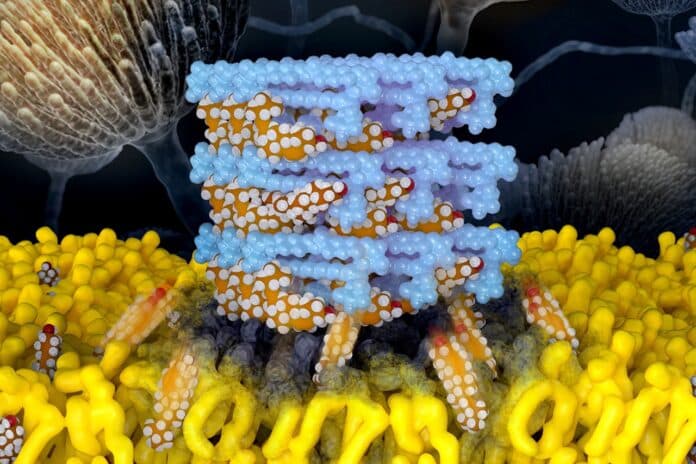
Burke’s team has spent years studying Amphotericin B (AmB) to create a derivative that can eliminate fungi without harming humans. Using a building block-based approach to molecular synthesis and collaborating with experts in molecular imaging tools, led by Professor Chad Rienstra at the University of Wisconsin-Madison, they uncovered AmB’s mechanism: it kills fungi by acting like a sponge, extracting ergosterol from fungal cells.
In their latest research, Burke’s team, again working with Rienstra’s group, found that AmB similarly harms human kidney cells by extracting cholesterol, the most common sterol in people. The researchers also revealed the atomic-level structure of AmB sponges when bound to both ergosterol and cholesterol.
Illinois graduate student Corinne Soutar, a co-first author of the paper, said, “The atomic resolution models were the key to zoom in and identify these very subtle differences in binding interactions between AmB and each of these sterols.”
“By understanding how Amphotericin B (AmB) works at a structural level, the researchers achieved a significant breakthrough. This insight allowed them to modify AmB, adjusting its binding properties to reduce interaction with cholesterol and, consequently, lower its toxicity,” explained Rienstra.
The team, consisting of Arun Maji, Agnieszka Lewandowska, and Corinne Soutar, conducted extensive testing on potential candidates for efficacy and toxicity in lab settings (in vitro) and living organisms (in vivo).
Armed with the insights from these studies, the Illinois team began creating and testing derivatives of AmB. These derivatives had slight changes in the area that binds to ergosterol and cholesterol while improving the ergosterol-removing process to maintain effectiveness.
With the support of collaborators and facilities at the Carl R. Woese Institute for Genomic Biology, along with Dr. Timothy Fan from the University of Illinois Veterinary Clinical Medicine, the researchers tested the most promising derivatives through a series of assessments: first in lab assays to quickly evaluate their ability to kill fungi, then in cell cultures, and finally in live mice to assess toxicity.
A standout molecule named AM-2-19 showed promising characteristics. Co-first author Arun Maji described it as kidney-friendly, resistant to resistance, and effective against a wide range of pathogens. Tested against over 500 clinically relevant species, AM-2-19 either matched or outperformed current antifungal drugs in efficacy. In screening for toxicity, the molecule proved safe in human blood and kidney cells. Mouse models of common fungal infections also demonstrated high efficacy.
Reflecting on the potential of AM-2-19, lead researcher Burke expressed excitement about decoupling effectiveness from toxicity. Previously known as “ampho-terrible,” AmB’s new derivative transforms it into something potentially “ampho-terrific.” While the researchers are optimistic, clinical studies are needed to confirm the molecule’s effectiveness in people.
In a move towards clinical use, the promising AM-2-19 has entered Phase 1 clinical trials after being licensed to Sfunga Therapeutics. The company also contributed to the research, and lead researcher Burke received consulting income and equity from them. The National Institutes of Health provided support for this work.
In conclusion, the newly discovered antifungal molecule demonstrates promising efficacy in eradicating fungi while exhibiting negligible toxicity in both human cells and mice. This breakthrough holds significant potential for developing safer and more effective antifungal treatments, addressing a critical need in medical therapeutics. Further research and clinical trials will be essential to validate and optimize the compound’s application, paving the way for its potential incorporation into future antifungal therapies.
Journal reference:
- Maji, A., Soutar, C.P., Zhang, J. et al. Tuning sterol extraction kinetics yields a renal-sparing polyene antifungal. Nature. DOI: 10.1038/s41586-023-06710-4.