University of Michigan researchers found a new plant chemistry that can make circular peptides. These molecules are essential for creating drugs that can attach to hard-to-target areas in the body. Circular peptides are a hopeful field in drug research.
A study by U-M College of Pharmacy researchers Lisa Mydy and Roland Kersten found how plants make circular peptides. Mydy discovered a new plant protein shape with unique chemistry never seen before. This protein can produce circular peptides, including one that could be used as an anti-cancer medicine.
Mydy is a postdoctoral research fellow in the Department of Medicinal Chemistry, “It’s fascinating. This type of discovery doesn’t happen too often.”
Mydy and her team examined how plants make specific circular peptides that could be used as medicine. They found a new protein shape that makes these circular peptides unique. Mydy called this discovery a new kind of chemistry we haven’t seen before.
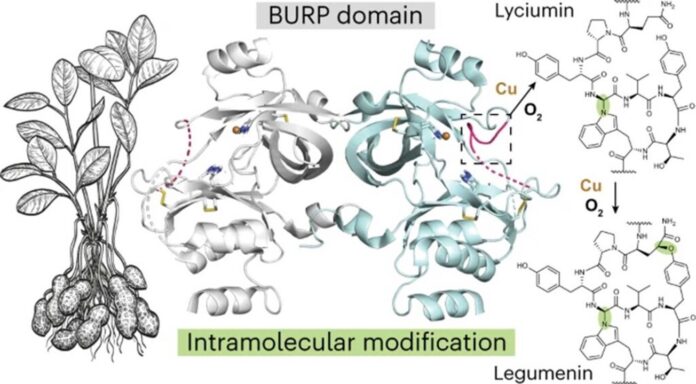
The researchers also studied a protein called AhyBURP found in peanut plant roots. This protein belongs to a USP-type group, part of the BURP-domain protein family.
Mydy said, “There was no experimental information on our protein AhyBURP. The only hint we had for the function was that the protein needed copper to cyclize a peptide.”
The research team used X-ray crystallography to study protein structures, utilizing the Advanced Photon Source at Argonne National Laboratory. During this process, they discovered that the protein AhyBURP utilizes copper and oxygen uniquely, a phenomenon still under investigation, according to Mydy.
Unlike most cyclic peptides, which require another enzyme to facilitate cyclization, AhyBURP can perform this process within the same protein. Mydy noted that while other copper-dependent proteins typically attach oxygen somewhere on the peptide, they didn’t observe this in AhyBURP, prompting further inquiry into why. She sees this as the first instance of such chemistry occurring with copper and oxygen within a protein.
The new protein discovery came from ongoing research in Kersten’s lab. Their lab, part of the U-M Natural Product Discovery Initiative, looks for new plant-based chemicals that could become drugs to treat diseases.
Kersten, an assistant professor of medicinal chemistry at the College of Pharmacy, explained that they use a modern method to find plant genes linked to new chemistry. This led them to discover the cyclic peptide products and their associated proteins.
These peptides are intriguing because their ability to form cycles makes them more organized and durable, which could make them effective drugs.
Many drugs, including those from living organisms, are cyclic, meaning they can bind to drug targets and stay intact in the body for a while. Nature has developed ways to create such cyclic molecules.
Kersten has found other compounds from the same protein family that can suppress lung cancer cells in lab tests. This raises hope that this discovery could be a future anti-cancer treatment.
Mydy said, “Now that we’ve identified the protein for one of the BURP-domain proteins, we can explore how it affects the chemical reaction to form cyclic peptides. Who is trained in structural biology and enzymology?
Understanding why this happens, and the structure involved is a fascinating puzzle. Being part of this discovery that could lead to effective medicines is exciting.
The discovery of the new plant protein fold, AhyBURP, and its ability to generate cyclic peptides represent a significant breakthrough in drug research. This finding holds promise for developing novel anti-cancer drugs. It highlights the importance of exploring natural sources for potential therapeutic agents.
Journal reference:
- Mydy, L.S., Hungerford, J., Chigumba, D.N. et al. An intramolecular macrocyclase in plant ribosomal peptide biosynthesis. Nature Chemical Biology. DOI: 10.1038/s41589-024-01552-1.