Diabetes is a common disorder that causes too high blood glucose levels. Left untreated, it can prompt serious medical issues like kidney failure, heart disease, and vision loss.
This disorder happens when there isn’t suffice insulin in the body or when insulin-incited responses are debilitated. Insulin ordinarily gives glucose access to cells for energy use; thus, without it, glucose develops in the blood. Lack of insulin cause due to the pancreatic beta cells, which ordinarily blend and emit insulin, have quit working effectively.
Defects in beta cells can lead to high glucose levels in the blood and, eventually, diabetes. A new study at the Okinawa Institute of Science and Technology Graduate University (OIST) and Riken Center of Integrative Medical Sciences, suggests that a protein called CNOT3 plays a vital role in regulating glucose levels. This protein was found to silence a set of genes that would otherwise cause insulin-producing cells to malfunction, which is related to the development of diabetes.
Dr. Dina Mostafa, former Ph.D. student in the Unit and first author of the paper published in Communications Biology, said, “Our results suggest that CNOT3 has a hand in this and plays a key role in maintaining normal beta-cell function.”
The protein CNOT3 helps to keep cells alive, healthy, and functioning correctly. It does this through several different mechanisms, such as producing the right proteins or suppressing specific genes.
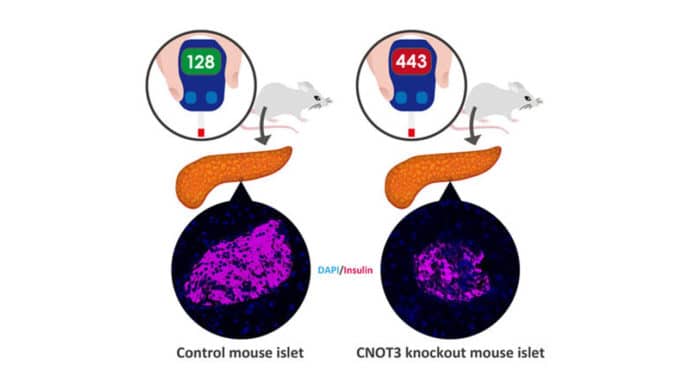
In this study, scientists observed whether CNOT3 expression differed in diabetic mice compared with non-diabetic mice. By looking at these islets, they found a significant decrease in the CNOT3 in the diabetic islets as opposed to the non-diabetic ones.
To additionally explore the protein’s capacity, the specialists hindered its production in the beta cells of otherwise normal mice. For about a month, the animals’ metabolism worked normally, however by the eighth week, they had built up an intolerance to glucose, and by 12 weeks, they full-blown diabetes.
Without CNOT3, the researchers found that some genes, which are typically switched off in beta cells, switch on and start to produce proteins. Under normal circumstances, these genes are silenced because once they switch on, they cause all kinds of problems for the beta cells, such as stopping them from secreting insulin in response to glucose.
Dr. Mostafa said, “We still don’t know that much about these kinds of genes, such as what their normal function is and the mechanism that’s involved in their silencing. So, it was very rewarding to find that CNOT3 in an important factor in keeping them switched off.”
Detail study of the cellular mechanisms behind this revealed an association between CNOT3 and the messenger RNA of these normally switched-off genes. Under normal circumstances, the mRNA of these genes hardly expresses. But once CNOT3 was removed, the scientists found that the mRNA was much more stable. Protein was produced from the stabilized mRNA, which has unfavorable effects on normal tissue function. This suggests that at least one way that these genes are kept switch off is through their mRNA’s destabilization, driven by CNOT3.
Journal Reference:
- Mostafa, D., Yanagiya, A., Georgiadou, E. et al. Loss of β-cell identity and diabetic phenotype in mice caused by disruption of CNOT3-dependent mRNA deadenylation. Commun Biol 3, 476 (2020). DOI: 10.1038/s42003-020-01201-y