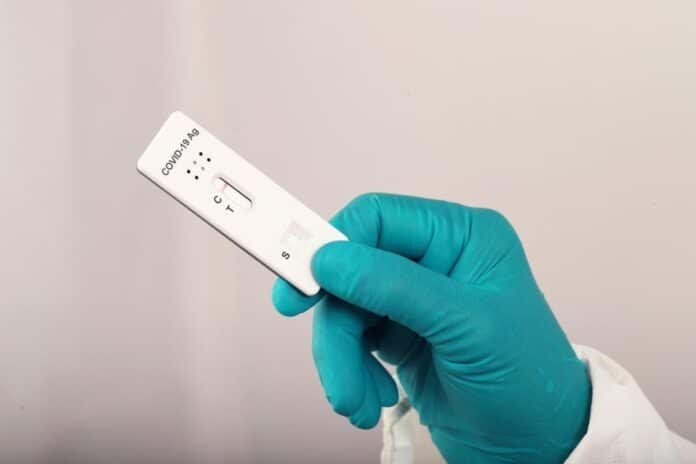
UNSW engineers developed biosensing tech for gene test strips, rivaling lab tests. During the pandemic, long queues for lab-based PCR tests caused delays. The new tech reduces inconvenience and logistics, providing faster COVID-19 results.
According to the work of UNSW Sydney biomedical engineers, test strips that offer quick, on-the-spot disease identification are as accurate as lab-based PCR tests. Dubbed “PCR in your pocket,” the technology has applications in the food business, biosafety, agriculture, and public health.
Senior researcher Professor Ewa Goldys with UNSW’s Graduate School of Biomedical Engineering said, “Not only can we easily detect specific gene sequences in a sample, but, unlike PCR, we can do it at room temperature using a test strip that looks exactly like a well-known RAT COVID test—you already know what to do with it. “So, no more” queuing for that PCR test in the future. Also, the cost is very low – currently less than a few dollars per test.”
According to study author Dr. Fei Deng, the new test strips may expedite the response to developing diseases such as mosquito-borne infections or lumpy skin ailments. They could also help locate endangered animal species and pinpoint areas for antibiotic resistance.
According to Dr. Deng, this has the potential to completely transform activities related to quarantine, biodiversity protection, and human and animal illness prevention. The gene-based assays redefined the biosensing landscape by being widely available to nearly anybody, anywhere, at any time.
According to Dr. Yi Li, a co-author of the work, the researchers created tiny DNA nano-circles, each measuring around two nanometers and carrying a brief sequence of the target DNA, such as the COVID-19 virus. No microscope large enough can see these nano-circles.
CRISPR/Cas proteins and the DNA nanocircles were combined with the material to be examined. The team configured these proteins, which are well-known for their part in the CRISPR/Cas gene editing technique that won the Nobel Prize, only to cut the nanocircles’ DNA when the targeted pathogen’s DNA activates them.
Dr. Li claims that the DNA nano-circles fragment, linearize, and turn into “fake targets” due to the interaction between the gene target and the programmed CRISPR/Cas protein. This procedure starts a chain of molecules.
Prof. Goldys mentions that the industry has responded positively to their innovation. The technology is being rolled out to Australian industries, with manufacturing planned locally. They aim to support RNA vaccine production.
The biosensing method has diverse applications: detecting invasive marine species for biosecurity, indicating threatened species in environmental samples, and detecting cancer cells.
The method successfully detected cancer mutations in a clinical setting. Prof. Goldys hopes this will lead to universal monitoring for patients undergoing cancer therapies.
The development of these test strips marks a significant advancement in gene-based diagnostics. They offer rapid and accurate disease detection capabilities. With broad applications across various sectors and promising clinical implications, this innovation holds the potential to revolutionize disease diagnostics and monitoring practices.
Journal reference:
- Deng, F., Li, Y., Yang, B. et al. Topological barrier to Cas12a activation by circular DNA nanostructures facilitates autocatalysis and transforms DNA/RNA sensing. Nature Communications. DOI: 10.1038/s41467-024-46001-8.