Human embryonic development has long captivated scientists and researchers, as it holds the key to unraveling the intricate mechanisms behind life’s origin and growth. Until recently, gaining a comprehensive understanding of the earliest stages of human embryogenesis has remained a formidable challenge. However, a groundbreaking new model has emerged, offering an unprecedented window into the remarkable journey of human embryonic development.
Recent research has unveiled an unparalleled opportunity to study the critical stage of gastrulation in human embryonic development, which occurs two to three weeks after conception. During this phase, embryonic cells undergo a transformative process, giving rise to specialized cells and initiating a remarkable surge of cellular diversity.

These cells serve as precursors for various cell types, including blood, tissue, and muscle. At the same time, the formation of primitive body axes takes shape. While investigating this process in the human-specific context has proven challenging, groundbreaking research now provides an unprecedented glimpse into this pivotal moment, offering invaluable insights into human development.

A groundbreaking approach to studying human embryonic development involves modeling it using stem cell technologies, addressing previous challenges researchers face. Traditional developmental models often lacked crucial supporting tissues for embryonic growth. However, a pioneering model incorporating both embryonic and extraembryonic components enables scientists to examine the intricate interactions between these parts during gastrulation stages.
This novel perspective offers valuable insights into the molecular and cellular processes, potentially unraveling the causes of pregnancy failures and congenital disorders. Published in Nature, the study led by researchers at Yale School of Medicine demonstrates an ethical and alternative means to explore the earliest stages of human growth using stem cell models, providing a glimpse into hidden aspects of early development that occur within the mother’s body, as highlighted by experts in the field.

Haifan Lin, Ph.D., Eugene Higgins Professor of Cell Biology, director of the Yale Stem Cell Center, and president of ISSCR said, “The Sozen and Smith groups have achieved a milestone in developing in vitro models to study the earliest stages of human development that are unfeasible yet so important for understanding health and disease. I commend their exceptional accomplishment and their sensitivity to ethical issues by limiting the model’s ability to develop further.”
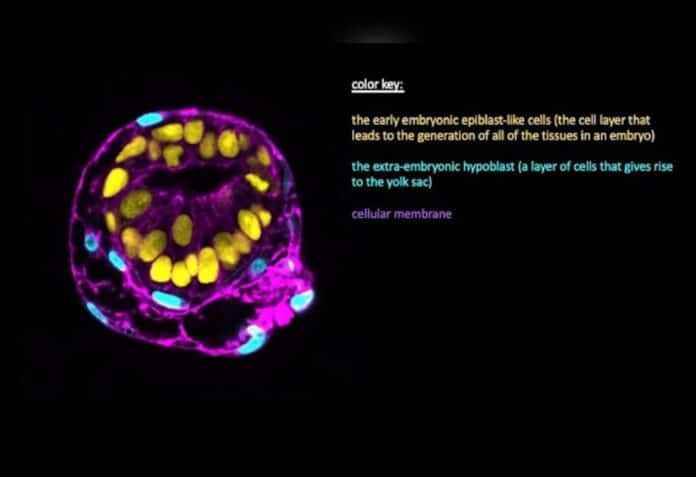
A recent study led by Yale School of Medicine researchers has developed a new model for studying embryonic development that includes both embryonic and extraembryonic tissues. This model addresses ethical concerns by lacking trophectodermal cells necessary for implantation and representing a developmental stage beyond the timeframe of implantation. The team grew embryonic stem cells in vitro and exposed them to conditions that led to self-organization and differentiation.
The cells formed two lineages: embryonic and extraembryonic precursors, with the latter serving as precursors for the yolk sac. The researchers gained mechanistic insights previously limited in the literature by analyzing the interactions and signals between these lineages. While the model cannot develop further or implant, it provides a reductionist approach to mimic and study aspects of natural development. It offers immense potential where ethical guidelines restrict the study of actual embryos.
The lack of accessibility to human embryos has created knowledge gaps in understanding human development, emphasizing the need for models that mimic human embryonic processes.
A new model developed by researchers offers direct insights into human gastrulation, a critical stage of development. This model provides access to the human system and enables large-scale research that is not feasible with human embryos. With an efficiency rate of over 70%, the model allows for exploring molecular pathways during gastrulation onset.
The researchers aim to investigate the developmental pathways and examine the potential links between failures during gastrulation stages and pregnancy loss or congenital disorders. The model’s unique features, including extra tissue, offer promising opportunities for in-depth analysis and advancing knowledge in human developmental biology.
Journal Reference:
- Pedroza, M., Gassaloglu, S.I., Dias, N. et al. Self-patterning of human stem cells into post-implantation lineages. Nature. DOI:10.1038/s41586-023-06354-4