Aqueous halides in charge-transfer-to-solvent (CTTS) states are good models for studying electron-transfer dynamics to the solvent. Solvent polarization and electronic excitation interact intricately in these systems. Despite multiple experimental investigations, it has been difficult to achieve a thorough knowledge of CTTS dynamics.
In a new study, EPFL scientists visualized the intricate interplay between electron dynamics and solvent polarization in this process. This is a significant step in understanding a critical process of many chemical phenomena, and it might be the first step to improving energy conversion technologies.
CTTS is like a dance of microbes where one electron from a dissolved material (like salt) emerges and becomes part of the water. This produces a “hydrated” electron, essential for several watery processes, including those necessary for life. Comprehending CTTS is crucial to understanding the motion of electrons in solutions.
In a recent study, EPFL researchers Jinggang Lan, Majed Chergui, and Alfredo Pasquarello examined the complex interactions between electrons and their solvent surroundings. The work was mostly done at EPFL, with Jinggang Lan’s final contributions made while he was a postdoctoral fellow at the Simons Center for Computational Physical Chemistry at New York University.
It carefully illustrated the dynamic interaction between the polarizing water molecules surrounding the escaping electron. This is a significant breakthrough in our comprehension of these intricate relationships.
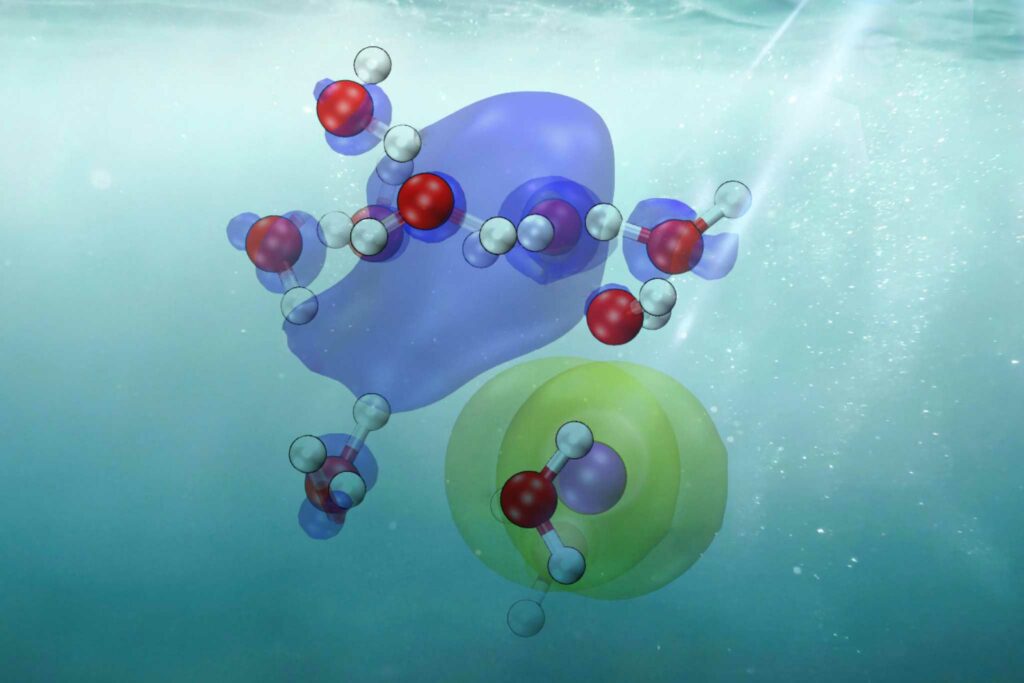
The group decided to work with iodide that has been dissolved in water, or “aqueous iodide,” because it makes comprehending how electrons enter surrounding water easier. Like table salt, iodide has simple internal movements, which facilitate research. This made it possible for scientists to see how the arrangement of the water molecules around the iodide affects how quickly the iodide releases an electron into the surrounding water.
Researchers used a complex method called ab initio molecular dynamics to simulate molecular behavior in a computer. This method uses quantum mechanics to calculate atomic movements and interactions based on fundamental physical principles, providing insight into the CTTS process. This “from the beginning” (Latin for “beginning”) approach allows precise predictions of the evolution of materials and molecules without depending on empirical facts for particle interactions.
Scientists were able to see and study the CTTS process in unprecedented detail by combining the ab initio approach with cutting-edge machine learning techniques. This allowed them to monitor the electron’s path from attachment to an iodide ion to becoming solvated or encircled and stabilized by water molecules.
Alfredo Pasquarello said, “The advance mostly rests at the fundamental level. The described mechanism involves a subtle interplay between electronic excitation and ionic polarization effects, which produce a sequence of configurations as revealed by our simulations.”
Jinggang Lan said, “Understanding charge transfer to solvent provides insights into the behavior of energy and electrons in chemical reactions, influencing a range from natural biological activities to the technology used in energy conversion.”
Journal Reference:
- Jinggang Lan, Majed Chergui, Alfredo Pasquarello. Charge Transfer to Solvent Dynamics in Aqueous Iodide. Nature Communications 21 March 2024. DOI: 10.1038/s41467-024-46772-0
