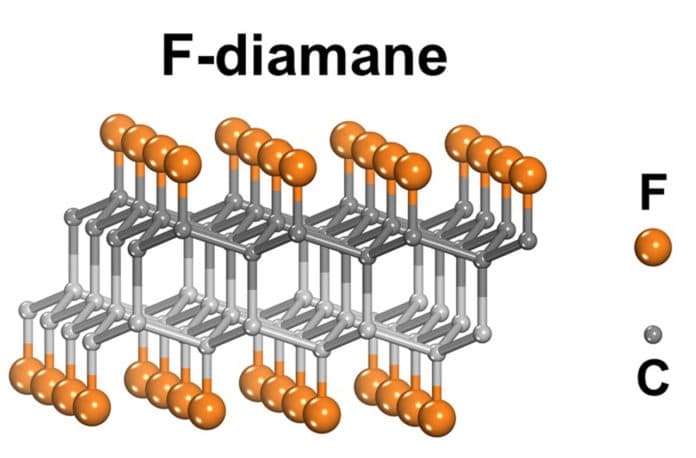
In a new experiment, UNIST scientists have successfully converted large-area bilayer graphene to the thinnest possible diamond-like material, under moderate pressure and temperature conditions. This adaptable, durable material initially acts as a wide-bandgap semiconductor, and subsequently has the potential for mechanical applications in nano-optics, nanoelectronics, etc.
Scientists actually invented a new method to promote the formation of diamane. The method involved exposing bilayer graphene to fluorine (F). Scientists then used vapors of xenon difluoride (XeF2) as the source of F. They got an ultra-thin diamond-like material, namely fluorinated diamond monolayer: F-diamane, with interlayer bonds and F outside.
For a more detailed description, the F-diamane synthesis was achieved by fluorinating large-area bilayer graphene on single-crystal metal (CuNi(111) alloy) foil, on which the needed type of bilayer graphene was grown via chemical vapor deposition (CVD).
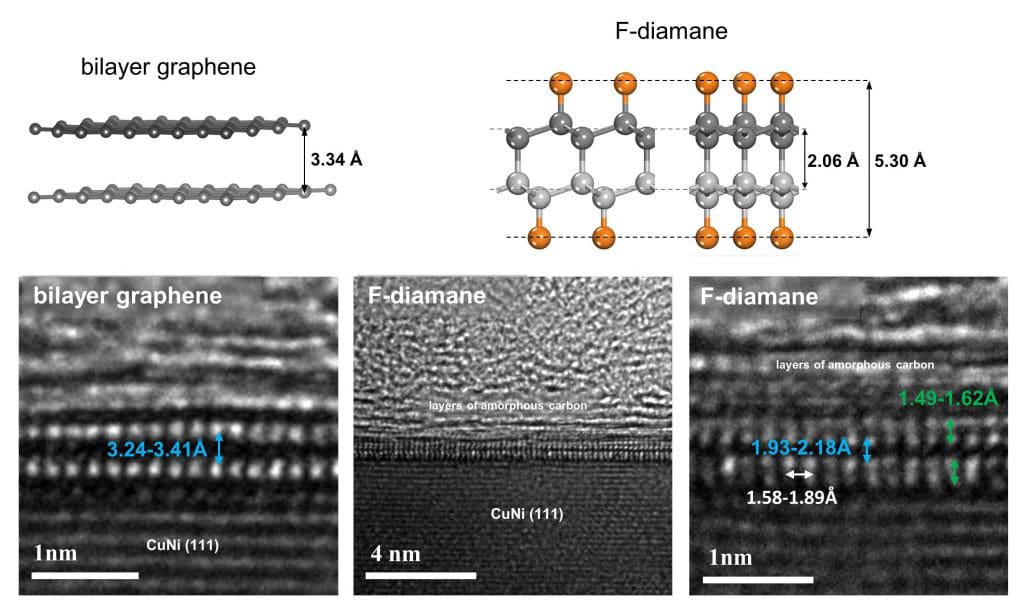
Conveniently, C-F bonds can be adequately described and recognized from C-C bonds. The group investigated the example after 12, 6, and 2-3 hours of fluorination. In light of the broad spectroscopic examinations and transmission electron microscopy, the scientists had the option to unequivocally show that the expansion of fluorine on bilayer graphene under certain well-defined and reproducible conditions brings about the arrangement of F-diamane. For instance, the interlayer space between two graphene sheets is 3.34 angstroms, yet is decreased to 1.93-2.18 angstroms when the interlayer bonds are shaped, as additionally anticipated by the hypothetical examinations.
Pavel V. Bakharev, the first author and co-corresponding author, said, “This simple fluorination method works at near-room temperature and under low pressure without the use of plasma or any gas activation mechanisms, hence reduces the possibility of creating defects.”
Ming Huang, one of the first authors, said, “Moreover, the F-diamane film could be freely suspended. We found that we could obtain a free-standing monolayer diamond by transferring F-diamane from the CuNi(111) substrate to a transmission electron microscope grid, followed by another round of mild fluorination.”
Distinguished Professor Ruoff notes that this work might spawn a worldwide interest in diamanes, the thinnest diamond-like films, whose electronic and mechanical properties can be tuned by altering the surface termination using nanopatterning and/or substitution reaction techniques. He further notes that such diamane films might also eventually provide a route to huge area single-crystal diamond films.
The findings of this research have been published in Nature Nanotechnology (IF 33.407) on December 10, 2019.