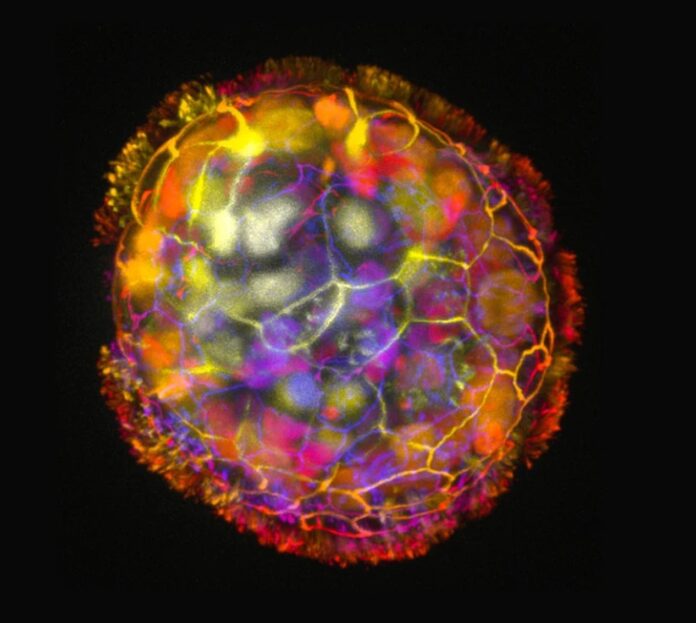
Scientists at Tufts and Harvard made tiny biological robots called Anthrobots using human tracheal cells. These tiny robots move and, in the lab, help neurons grow in damaged areas. The researchers hope to use similar biobots made from patients’ cells to heal and treat diseases.
These robots are as small as a human hair to the tip of a sharpened pencil. They self-assemble in the lab dish. Also, they don’t require tweezers or scalpels to give them shape.
Before this, scientists in the laboratories of Michael Levin, Vannevar Bush, Professor of Biology at Tufts University School of Arts & Sciences, and Josh Bongard at the University of Vermont made tiny living robots called Xenobots from frog cells. These Xenobots could do many things like move, collect stuff, remember things, heal, and make more of themselves. Now, they’re trying to see if they can do similar things using cells from other species, not just frogs.
In this study, Levin and PhD student Gizem Gumuskaya discovered they could make these tiny bots from adult human cells without changing their genes. These new bots can do more than those made from frog cells. This discovery helps answer a big question: what rules govern how cells assemble and work together in the body, and can the cells be taken out of their natural context and recombined into different “body plans” to carry out other functions by design?
In this case, scientists allowed human cells to regenerate and discover new methods to form structures and functions after decades of quiet life in the trachea. They were keen to see what cells can do besides create default features in the body.
PhD student Gizem Gumuskaya said, “By reprogramming interactions between cells, new multicellular structures can be created, analogous to the way stone and brick can be arranged into different structural elements like walls, archways, or columns.”
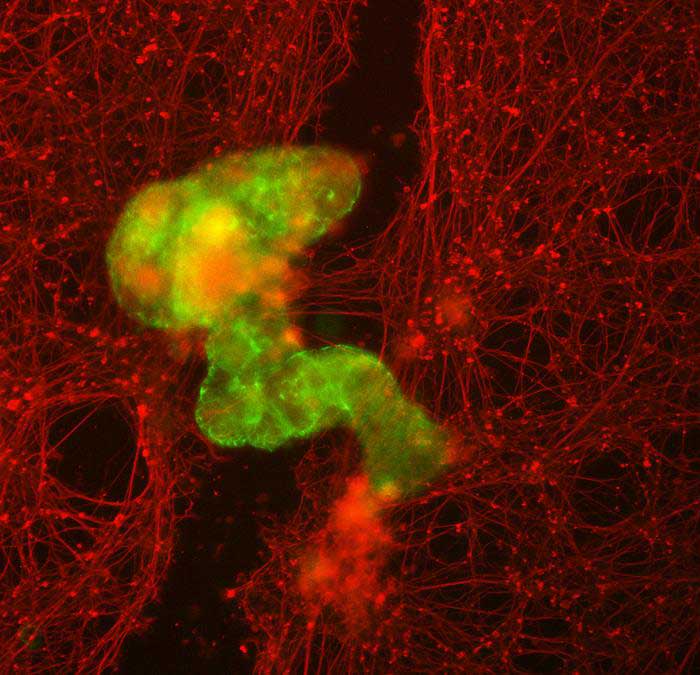
The scientists discovered that these cells could make new shapes and move differently on the surface of human neurons in a lab dish. They also helped new neurons grow when gaps were caused by scratching the cell layer. They made a group of Anthrobots called a “superbot,” and neurons grew under it, although they’re not sure exactly how.
Levin said, “The cellular assemblies we construct in the lab can have capabilities beyond what they do in the body. It is fascinating and completely unexpected that normal patient tracheal cells, without modifying their DNA, can move independently and encourage neuron growth across a region of damage. We’re now looking at how the healing mechanism works and asking what else these constructs can do.”
How did scientists make Anthrobots?
Each Anthrobot starts as a single cell taken from an adult’s trachea, a part of the airway. These cells have tiny hair-like parts called cilia that move back and forth, helping the cells push out small particles from the air passages. In the lab, these cells naturally form small groups called organoids.
Scientists made the cilia face outward on these groups, and soon, the organoids started moving around like boats with oars. They saw different shapes and movements, the first important feature of these tiny robots. The scientists think adding more features to the Anthrobots, like other types of cells, could make them do specific jobs in the body or help build tissues in a lab.
With the help of Simon Garnier at the New Jersey Institute of Technology, the team studied the different Anthrobots produced. They found that the bots came in a few different shapes and sizes, ranging from 30 to 500 micrometers (as thin as a human hair to the point of a sharp pencil). This makes them fit between very small nanotechnology and larger devices.
Some were football-shaped, uneven, and had sporadic or only partially covered cilia, while others were spherical and wholly covered in cilia. They moved in tight circles, in straight lines, in combination with one another, or just sat there wriggling. The cilia-covered spherical ones were typically wigglers. The Anthrobots with an uneven distribution of cilia preferred to travel in straight or curved patterns for longer distances. In experimental settings, they typically survived for 45–60 days before starting to decompose naturally.
Scientists want Anthrobots to be used for healing, so they tested if the bots could help fix wounds. They grew a 2D layer of human neurons in the lab and made a ‘wound’ by scratching it with a thin metal rod. To ensure the Anthrobots stayed near the gap, they made ‘superbots,’ a group of Anthrobots that naturally form when they are in a small space. These superbots were mostly made of circlers and wigglers so that they would stay close to the open wound.
Some might think that the Anthrobots would need gene changes to help neural growth, but surprisingly, the normal Anthrobots caused significant regrowth. They made a bridge of neurons as thick as the healthy cells on the plate, while no neurons grew where there were no Anthrobots. In the simple lab dish world, Anthrobot groups helped the neural tissue heal effectively.
Scientists noted, “Further development of the bots could lead to other applications, including clearing plaque buildup in the arteries of atherosclerosis patients, repairing spinal cord or retinal nerve damage, recognizing bacteria or cancer cells, or delivering drugs to targeted tissues. The Anthrobots could, in theory, assist in healing tissues while also laying down pro-regenerative drugs.”
Gumuskaya explained that cells have the innate ability to self-assemble into larger structures in specific fundamental ways.
“The cells can form layers, fold, make spheres, sort and separate themselves by type, fuse together, or even move. Two important differences from inanimate bricks are that cells can communicate with each other and create these structures dynamically. Each cell is programmed with many functions, like movement, secretion of molecules, detection of signals, and more. We are just figuring out how to combine these elements to create new biological body plans and functions—different than those found in nature.”
The scientists can build the bots by utilizing the naturally flexible rules of cellular assembly. Still, they can also learn more about how natural body plans come together, how the environment and genome interact to form tissues, organs, and limbs, and how regenerative treatments can help restore them.
Journal Reference:
- Gizem Gumuskaya, Pranjal Srivastava, Ben G. Cooper, Hannah Lesser, Ben Semegran, Simon Garnier, Michael Levin. Motile Living Biobots Self-Construct from Adult Human Somatic Progenitor Seed Cells. Advanced Science. DOI: 10.1002/advs.202303575