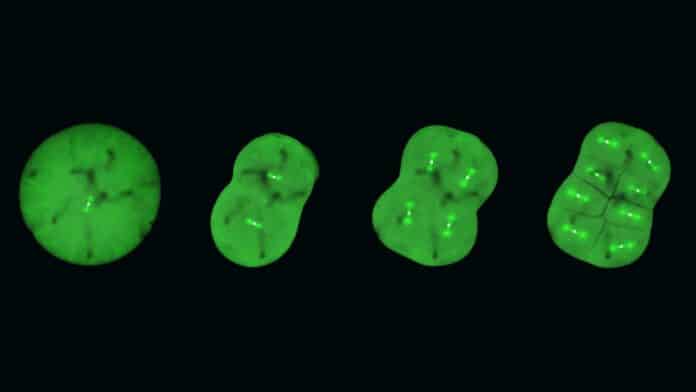
In mitosis, a cell replicates its chromosomes and then segregates them. The intricate dynamics of mitosis are well-known in somatic cells but remain elusive in embryonic cells.
Studying embryonic mitosis is very difficult as live functional analyses and -imaging of experimental embryos are technically limited.
Now, scientists from the Cell Division Dynamics Unit at the Okinawa Institute of Science and Technology (OIST), collaborating with scientists from the Universities of Nagoya, Tohoku, and the National Institute of Genetics, have combined novel imaging techniques to answer questions about embryonic mitosis. Those techniques were CRISPR/Cas9 genome editing technology, a modern protein-knockdown system, and medaka, or Japanese rice fish (Oryzias latipes).
Their time-lapse videos contribute to the resolution of significant mysteries about the even division of chromosomes during early cell division in embryos. Simultaneously, they are indicating future directions for scientific inquiry.
Professor Tomomi Kiyomitsu, senior author of the study, describes the timelapses as “beautiful, both on their own and because they lay a new foundation for elucidating embryonic mitosis.”
A critical stage in embryonic mitosis is the alignment and even division of the chromosomes, which contain all of the cell’s genetic information, into daughter cells. The mitotic spindle enters the picture at this point. Microtubules are long protein fibers that aid cell shape and material transport; they make up the mitotic spindle. These microtubules join the chromosomes in the center of the spindle after extending from opposite ends. During cell division, the spindle ensures that duplicated chromosomes are appropriately collected and distributed equally among the daughter cells.
A protein known as Ran-GTP is one of the many elements that affect how the spindle forms. Since female reproductive cells lack centrosomes and organelles that assemble microtubules, Ran-GTP is especially crucial during cell division in these cells, but not in smaller somatic cells that do contain centrosomes. Nevertheless, whether Ran-GTP is required for spindle assembly in early vertebrate embryos has yet to be shown. Despite having centrosomes, these embryos are unique because of things like more giant cells, which might have an impact on the process in a different way.
Fish embryos are more accessible to track since they are transparent and grow into a single-layer sheet, unlike early mammalian embryos. Medaka fish can withstand a wide range of temperatures, lay eggs daily, and have a small genome, making them an excellent choice for the researchers’ investigation. They are ideal for long-term, live timelapse photography since they can endure at room temperature.
Medaka fish make ideal candidates for CRISPR/Cas9 genome editing. By genetically altering these fish to make their embryonic cells glow, researchers could observe the dynamics of specific proteins involved in cell division.
Upon examining these genetically altered live medaka embryos, the scientists discovered that early embryos differentiate from other cell types during cell division by forming distinct spindles. Additionally, they found that whereas Ran-GTP is essential for spindle formation in early embryos, its significance decreases with the later development of the embryos. This might be because, as cells shrink during development, the spindle’s structure varies, although further investigation is required to verify this.
According to the researchers, the spindle assembly checkpoint, a process found in most other cells, is absent from early embryonic cells. This checkpoint checks chromosome alignment to ensure it is correct before cell division.
Professor Kiyomitsu surmises, “The checkpoint is not active, and yet the chromosome segregations are still very accurate. This could be explained by the fact that embryonic cells need to divide very quickly, but it is something that we want to study further.”
Though Professor Kiyomitsu and the team believe this is just the beginning, studying the early embryos of medaka fish and genetically manipulating them has revealed crucial insights into embryonic cell division. They are fascinated by issues such as why early embryonic cells lack a spindle assembly checkpoint and why the function of Ran-GTP diminishes during embryonic development. The symmetry they see in cell division—how the spindle divides cells uniformly and always aligns in the middle of the cell—also fascinates them.
The group intends to increase the number of genetically altered medaka fish lines to further their research beyond time-lapse observations. These lines will be used to advance genome editing techniques and provide models for the study of embryonic cells. They hope to investigate the evolution of spindle assembly and embryonic divisions in the future and see if these conclusions hold true for other animals. This deeper comprehension may provide light on human embryonic development as well as aid in the identification and management of infertility problems.
Through this study, researchers laid out a strong foundation and opened up a new frontier.
Journal Reference:
- Kiyomitsu, A., Nishimura, T., Hwang, S.J. et al. Ran-GTP assembles a specialized spindle structure for accurate chromosome segregation in medaka early embryos. Nat Commun 15, 981 (2024). DOI: 10.1038/s41467-024-45251-w