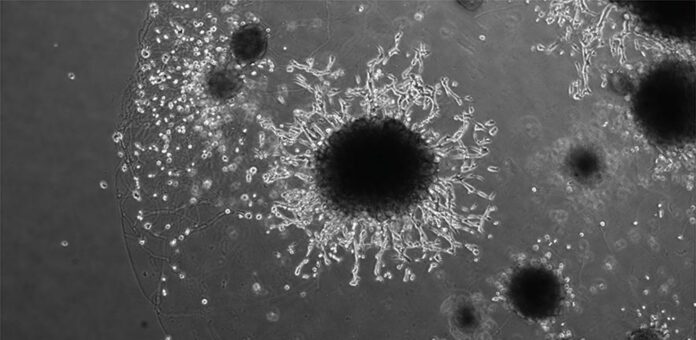
Scientists grew ‘mini-placentas’ in the lab, providing insights into placental development and its interaction with the womb lining. Understanding these processes is crucial for addressing pregnancy disorders like pre-eclampsia. Most pregnancy issues stem from early placental development, a challenging phase to study.
The study, published in Cell Stem Cell, demonstrates the possibility of experimenting with developing human placentas, offering a proactive approach to understanding and potentially treating major pregnancy disorders.
Successful pregnancy relies on crucial interactions between endometrial and placental cells. These interactions ensure an adequate maternal blood supply to support fetal growth. Complications, like pre-eclampsia (affecting 6 in 100 first pregnancies), arise when these interactions falter, posing risks to both mother and baby due to high blood pressure during pregnancy.
Professor Ashley Moffett from the Department of Pathology at the University of Cambridge said: “Most of the major disorders of pregnancy – pre-eclampsia, stillbirth, growth restriction, for example – depend on failings in the way the placenta develops in the first few weeks. This process is complicated to study – the period after implantation, when the placenta embeds itself into the endometrium, is often described as a ‘black box of human development.’
“Over the past few years, many scientists – including several at Cambridge – have developed embryo-like models to help us understand early pre-implantation development. But further development is impeded because we understand so little about the interactions between the placenta and the uterus.”
Professor Moffett and team used ‘mini-placentas,’ known as trophoblast organoids, as a cellular model for early pregnancy. These organoids closely mimic the early placenta, even triggering positive results on pregnancy tests. The Friedrich Miescher Institute and Wellcome Sanger Institute researchers aim to understand reproductive disorders.
Prior research identified genes influencing conditions like pre-eclampsia. Notably, uterine natural killer cells play a vital role in mediating interactions between the endometrium and placental cells, contributing to our understanding of early pregnancy.
In a recent study, Professor Moffett’s team applied proteins from uterine natural killer cells to trophoblast organoids, mimicking conditions for placental implantation. They identified crucial proteins for organoid development, essential for successful implantation. These proteins facilitate the placenta’s invasion into the uterus and the transformation of the mother’s arteries.
Professor Moffett highlighted the uniqueness of this process, where cells from the baby invade and transform the mother’s arteries. If this invasion falters, it hampers blood flow, leading to nutrient and oxygen deprivation for the baby, causing problems later in pregnancy.
Prof. Moffett’s team used proteins from uterine natural killer cells in a study on trophoblast organoids, replicating conditions for placental implantation. They found vital proteins crucial for organoid development, aiding successful implantation. These proteins help the placenta invade the uterus and transform the mother’s arteries. This unique process, where baby cells change the mother’s streets, is vital for proper blood flow. If this process fails, it can result in insufficient nutrients and oxygen for the baby, leading to complications later in pregnancy.
This study marks a significant step forward in unraveling the complexities of pre-eclampsia and pregnancy disorders. The utilization of ‘mini-placentas’ as a research model offers a promising avenue for continued exploration and understanding of reproductive health, with potential implications for improving maternal and fetal outcomes.
Journal reference:
- Qian Li, Andrew Sharkey, et al., Human uterine natural killer cells regulate differentiation of extravillous trophoblast early in pregnancy. Cell Stem Cell. DOI: 10.1016/j.stem.2023.12.013.