Amorphous materials are those materials made of plastic or glass. The atoms and molecules in amorphous materials never stack together to form crystals when cooled.
Although glass and plastic are often considered “solids,” they remain in a state better described as a supercooled liquid that flows very slowly. Although these “glassy dynamic” materials are common daily, scientists have long been baffled by how they become rigid at the microscopic level.
Scientists at the Lawrence Berkeley National Laboratory (Berkeley Lab) of the Department of Energy have found molecular activity in supercooled liquids indicative of a hidden transition between a liquid and a solid.
Their expanded knowledge relates to common materials like plastic and glass. It could aid scientists in creating new amorphous materials for application in additive manufacturing, medical devices, and drug delivery.
The scientists specifically explained why the molecules in these materials, when cooled, stay disordered like a liquid until turning toward a solid-like state at a certain temperature known as the onset temperature – effectively becoming so viscous that they barely move. This phase transition, the commencement of rigidity, distinguishes supercooled liquids from normal liquids.
Kranthi Mandadapu, a staff scientist in Berkeley Lab’s Chemical Sciences Division and professor of chemical engineering at the University of California, Berkeley, said, “Our theory predicts the onset temperature measured in model systems and explains why the behavior of supercooled liquids around that temperature is reminiscent of solids even though their structure is the same as that of the liquid.”
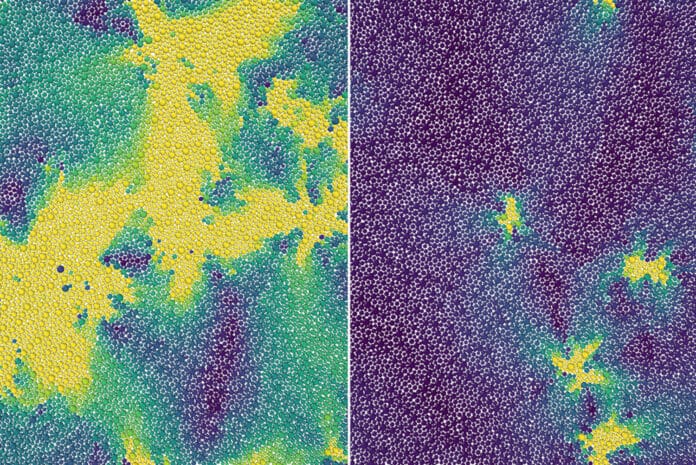
Any supercooled liquid constantly changes between various molecular arrangements, causing excitations or localized particle motions. The excitations in a 2D supercooled liquid were handled as flaws in a crystalline solid in Mandadapu, postdoctoral researcher Dimitrios Fraggedakis, and graduate student Muhammad Hasyim’s suggested theory.
They suggest that every instance of a bound pair of flaws split apart into an unbounded pair as the temperature of the supercooled liquid increased to the onset temperature. It was precisely at this temperature that faults unbound, causing the system to lose stiffness and behave more like a normal liquid.
Mandadapu said, “The onset temperature for glassy dynamics is like a melting temperature that ‘melts’ a supercooled liquid into a liquid. This should be relevant for all supercooled liquids or glassy systems.”
Other crucial aspects of glassy dynamics were also captured by theory and simulations, such as the finding that only a small number of particles moved briefly while most of the liquid stayed frozen.
Mandadapu said, “The whole quest is to understand microscopically what separates the supercooled liquid and a high-temperature liquid.”
Scientists are looking forward to extending their model to 3D systems to explain how localized motions lead to further nearby excitations resulting in the relaxation of the entire liquid. Together, these elements might offer a dependable microscopic view of how glassy dynamics evolve in a way that is consistent with cutting-edge data.
Mandadapu said, “It’s fascinating from a basic science point of view to examine why these supercooled liquids exhibit remarkably different dynamics than the regular liquids we know.”
Journal Reference:
- Dimitrios Fraggedakis, Muhammad Hasyim and Kranthi Mandadapu. Inherent-state melting and the onset of glassy dynamics in two-dimensional supercooled liquids. PNAS. DOI: 10.1073/pnas.2209144120