Amyloid plaques have long been suspected as the root cause of Alzheimer’s, but recent studies suggest that oligomers — floating molecules with repeating peptide units — do far more damage.
Soluble oligomers are the leading cause of neuronal degeneration because these oligomers are toxic to neurons. These oligomers are indeed connected with Alzheimer’s pathology, so there is a requirement for devices that will enable scientists to examine them.
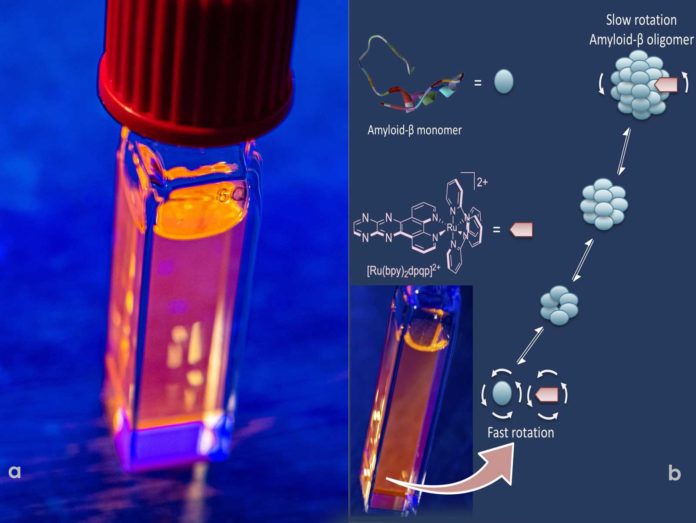
Scientists from Rice University have recently demonstrated a way to track the formation of solvent amyloid beta-peptide aggregates implicated at the beginning of Alzheimer’s disease. Scientists have developed a ruthenium-based fluorescent complex that binds to soluble, oligomeric amyloid-beta peptides. As the peptides come together to form the large biomolecules called oligomers, the fluorescent additive binds and labels them.
Angel Martí, an associate professor of chemistry, of bioengineering and materials science and nanoengineering, said, “Oligomers are “virtually invisible” to Thioflavin T dyes commonly used to tag amyloid fibrils in lab studies. The ruthenium complexes solve that problem.”
“The complexes take advantage of fluorescence anisotropy, in which the fluorescent response is polarized, glowing brighter in some directions than others. It’s an ancient technique related to the rotation of molecules. When the molecule is in solution, it moves and is constantly rotating. When it’s small, it rotates very fast, and the anisotropy is nearly zero.”
“But when the same probe binds to a large macromolecule, it rotates more slowly. That’s how we know we have oligomers, and then we can track their growth and propagation.”
During experiments, scientists found that oligomers form in solution at different temperatures over hours. Cold solutions slow the process, but at body temperatures, oligomers form very fast and in large amounts. The speed at which they form at physiological temperatures is remarkable.
Scientists also used its tests to perceive how neuroblastoma cells were influenced progressively when infused with amyloid-beta peptides. They uncovered just 60% of cells infused with oligomers remained viable, while those treated with amyloid fibrils and monomers had higher suitability, about 80%, proposing the oligomers are indeed toxic.
Marti said, “For now the ruthenium probes are meant for use only in the laboratory. It will be difficult to use these in the brain because there’s too much scattering of light. They are made to take advantage of polarized light, and scattering would dampen that.”
“But as a lab tool, they will allow researchers around the world to test the effects of other molecules on the rate of oligomer formation, and that’s a big deal. They can quickly see if drug delays or halts the formation of oligomers.”
Rice graduate student Bo Jiang is the lead author of the paper. Co-authors are Rice graduate student Ashleigh Smith McWilliams; undergraduate Andrea Augustine; Rice alumni Nathan Cook, now an instrumentation specialist in the Department of Chemistry at Williams College in Williamstown, Mass., Amir Aliyan, now a researcher at Khatam University in Tehran, Iran, and Rodrigo Maldonado, now a graduate student at Northwestern University; Ghibom Bhak and Javier Montenegro of the Universidade de Santiago de Compostela, Spain; Erick Flores and Fernando Godoy of the Universidad de Santiago de Chile; and Nicolas Mendez, Mohammad Shahnawaz and Ines Moreno-Gonzalez of The University of Texas Health Science Center at Houston.
Details of the work appear in the Journal of the American Chemical Society.