A research team at the Weizmann Institute of Science has developed entire models of human embryos from lab-cultivated stem cells. And they even succeeded in growing these embryos outside of the womb.
This new study could pave the way for future work on infertility, drug development, and tissue growth for transplants, as well as allow researchers to see inside the dramatic initial weeks of embryonic development.
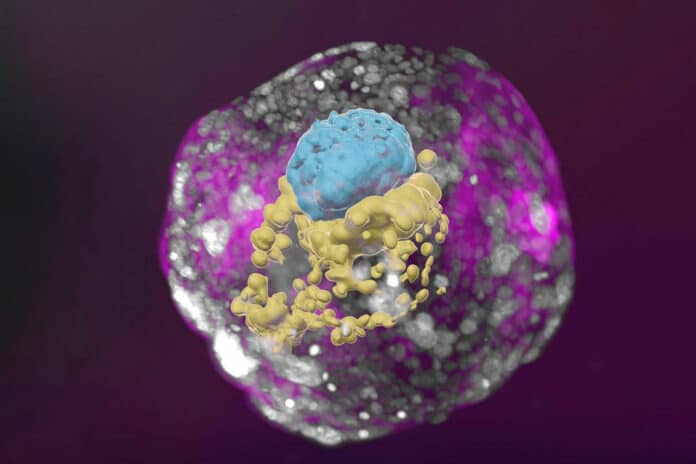
The placenta, yolk sac, chorionic sac, and other exterior tissues that guarantee the models’ dynamic and sufficient growth were all present in these synthetic embryo models and other compartments and structures typical of this stage.
Unlike previously developed human embryo models, with their authentic complexity, these new human embryo models may offer unprecedented opportunities to shed new light on the embryo’s mysterious beginnings. It closely mimics the development of a real human embryo, particularly the emergence of its exquisitely fine architecture.
The team led by Hanna expanded on their prior expertise in producing synthetic mouse embryo models using stem cells. The scientists did not use fertilized eggs or a womb, just like in that study. Instead, they began with pluripotent stem cells from humans, which can differentiate into most types of cells but not all of them. Some were from mature skin cells that had reverted to their “stemness.” Others were the offspring of human stem cell lines grown in a lab for several years.
To further turn the clock back, the scientists used Hanna’s novel method to reprogram pluripotent stem cells. They did this by returning them to an even earlier state known as the naive state, where they could become anything, specializing in any cell type. This stage occurs when the natural human embryo inserts itself in the womb, or day 7 for the human embryo.
Hanna’s team was the first to describe how to make human naive stem cells back in 2013. Over the years, they continued to refine these techniques, which are the foundation of the current effort.
Scientists divided the cells into three groups. The embryo’s precursor cells were not altered in any way. To turn on specific genes and cause the cells in each of the other groups to differentiate into one of the three tissue types required to support the embryo—the yolk sac, placenta, or the extraembryonic mesoderm membrane that ultimately gives rise to the chorionic sac—certain chemicals were applied to the cells in each of the other groups.
Hanna said, “Soon after being mixed under optimized, specifically developed conditions, the cells formed clumps, about 1 percent of which self-organized into complete embryo-like structures.”
“An embryo is self-driven by definition; we don’t need to tell it what to do – we must only unleash its internally encoded potential. It’s critical to mix in the right kinds of cells initially, which can only be derived from naïve stem cells with no developmental restrictions. Once you do that, the embryo-like model itself says, ‘Go!'”
The stem cell-based embryo-like structures, also known as SEMs, grew normally for 8 days outside the womb, reaching a developmental stage corresponding to day 14 in forming a human embryo. Natural embryos reach that stage when they establish the internal structures needed to move on to the following phase: the development of the bodily organ progenitors.
Scientists discovered an uncanny structural similarity between the stem cell-derived embryo models and the natural human embryos at the corresponding stage when they compared the inner organization of the models to illustrations and microscopic anatomy sections in classic embryology atlases from the 1960s. Every storage space and auxiliary support was present and positioned, sized, and shaped correctly.
Even the cells that produce the hormone used in pregnancy tests were present and functioning properly, as evidenced by the positive results of a commercial pregnancy test when the scientists put secretions from these cells into it.
Hanna says, “This implied that their models faithfully emulated the process by which an early embryo gains all the structures it needs for beginning its transformation into a fetus. Many pregnancy failures occur in the first few weeks, often before the woman knows she’s pregnant.”
“That’s also when many congenital disabilities originate, even though they tend to be discovered much later. Our models can be used to reveal the biochemical and mechanical signals that ensure proper development at this early stage and the ways in which that development can go wrong.”
The study has already revealed a finding that could lead to a new line of inquiry into the failure of early pregnancies. The scientists found that internal features like the yolk sac fail to grow properly in the embryo if the placenta-forming cells are not fully encircling the embryo by day 3 of the protocol (equivalent to day 10 in natural embryonic development).
Hanna says, “An embryo is not static. It must have the right cells in the right organization, and it must be able to progress – it’s about being and becoming. Our complete embryo models will help researchers address the most basic questions about what determines its proper growth.”
This moral approach to unraveling the riddles of the earliest phases of embryonic development may pave the way for new lines of inquiry. It might shed light on the origins of some birth abnormalities and infertility types. It might result in novel techniques for developing organ and tissue transplantation.
It might also provide a workaround for tests that can’t be done on live embryos, like figuring out how exposure to medicines or other substances affects fetal development.
Journal Reference:
- Oldak, B., Wildschutz, E., Bondarenko, V. et al. Complete human day 14 post-implantation embryo models from naïve ES cells. Nature (2023). DOI: 10.1038/s41586-023-06604-5