Secreted proteins mediate essential physiological processes. Understanding the spatial and temporal dynamics of protein secretion at a single-cell level is essential to understanding numerous biological systems relevant to health and disease. However, currently available methods for studying protein secretion at the single-cell level lack spatial resolution.
Hence, scientists at Washington University in St. Louis devised an approach to visualize the proteins secreted by cells with stunning resolution. They developed the FluoroDOT assay to see and measure proteins secreted by a single cell in about 30 minutes.
When tested, the FluoroDOT assay is found to be versatile, low cost, and adaptable to any laboratory setting. Plus, it can potentially offer a more comprehensive look at these proteins than the widely used existing assays.
Existing methods are limited in sensitivity and can take up to 24 hours to process. The novel method is different from those methods as it uses a plasmonic-fluor, a plasmon-enhanced nano label developed in Singamaneni’s lab that is 16,000 times brighter than conventional fluorescence labels and has a signal-to-noise ratio nearly 30 times higher.
Srikanth Singamaneni, the Lilyan & E. Lisle Hughes Professor of Mechanical Engineering & Materials Science at the McKelvey School of Engineering, said, “Plasmonic-floors are composed of metal nanoparticles that serve as an antenna to pull in the light and enhance the fluorescence emission of molecular fluorophores, thus making it an ultrabright nanoparticle.”
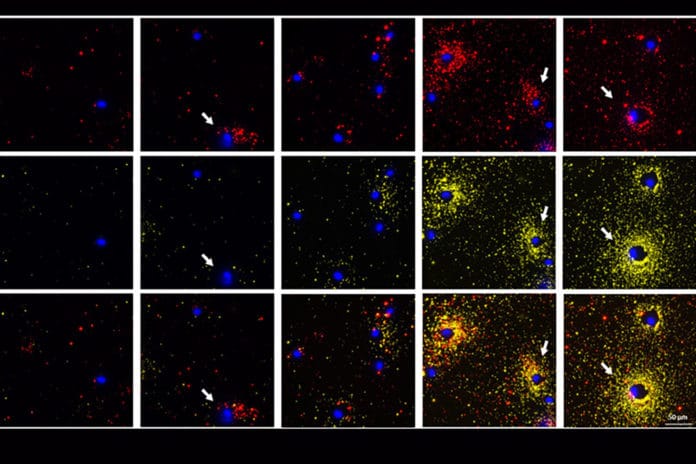
Plasmonic flours have ultrabright emission that allows extremely small quantities of secreted protein, which they cannot do in existing assays. Using a custom-built algorithm also allows digitally quantifying the high-resolution signals using the number of particles, or dot pattern, per cluster or spot.
Anushree Seth, a former postdoctoral scholar in Singamaneni’s lab, said, “Using a simple fluorescence microscope, we can simultaneously image a cell along with the spatial distribution of the proteins secreted around it. We saw interesting secretion patterns for different cell types. This assay also enables concurrent visualization of two types of proteins from individual cells. When the multiple cells are subjected to the same stimuli, we can distinguish the cells that are secreting two proteins simultaneously from the ones that are only secreting one protein or are not secreting at all.”
Scientists validated their method by using proteins secreted from human and mouse cells, including immune cells infected with Mycobacterium tuberculosis.
Philips said, “When Mycobacterium tuberculosis infects immune cells, those cells respond by secreting important immune proteins, called cytokines. But not all cells respond to infection the same way. The FluoroDOT assay allowed us to see how individual cells in a population respond to infection — to see which cells are secreting and in which direction. This was not possible with the older technology.”
Journal Reference:
- Anushree Seth, Ekansh Mitta, et al. High-resolution imaging of protein secretion at the single-cell level using plasmon-enhanced FluoroDOT assay. Cell Reports Methods, Augst 2022. DOI: 10.1016/j.crmeth.2022.100267