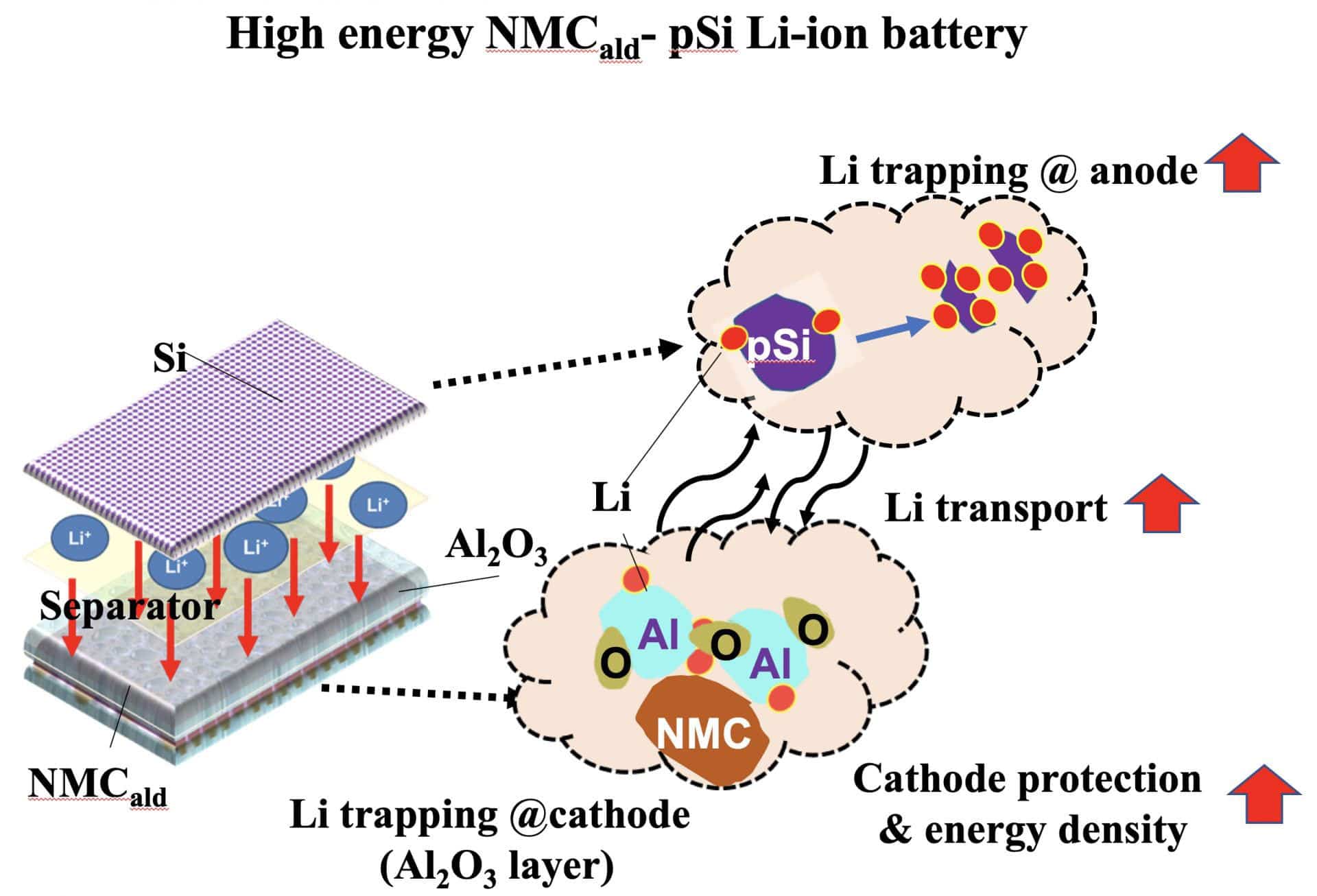
Conventional lithium-ion batteries use graphite-based anodes that have a capacity of under 400 milliamp hours per gram (mAh/g), yet silicon anodes have possibly ten times that limit. That accompanies a drawback: Silicon grows as it composites with lithium, stressing on the anode.
Maximum capacity puts a lot of stress on the material, so scientists from Rice University have discovered a mechanism by which lithium gets trapped in batteries, thus limiting the number of times it can be charged and discharged at full power. This is a strategy to get capacity without the same degree of stress.
Yet, that characteristic doesn’t hope that in certain circumstances, such batteries could be perfect.
The Rice lab of chemical and biomolecular engineer Sibani Lisa Biswal found a sweet spot in the batteries that, by not maximizing their storage capacity, could give consistent and stable cycling to applications that need it.

Scientists tested their idea of pairing the porous, high-capacity silicon anodes (instead of graphite) with high-voltage nickel manganese cobalt oxide (NMC) cathodes. The full cell lithium-ion batteries exhibited stable cyclability at 1,000 mAh/g over hundreds of cycles.
Some cathodes had a 3-nanometer layer of alumina (applied via atomic layer deposition), and some did not. Those with the alumina coating protected the cathode from breaking down in the presence of hydrofluoric acid, which forms if even minute amounts of water invade the liquid electrolyte. Testing showed the alumina also accelerated the battery’s charging speed, reducing the number of times it can be charged and discharged.
Postdoctoral fellow Anulekha Haridas said, “There appears to be extensive trapping as a result of the fast lithium transport through alumina. This is the first report of the alumina itself absorbing lithium until saturated. At that point, she said, the layer becomes a catalyst for fast transport to and from the cathode.”
“This lithium-trapping mechanism effectively protects the cathode by helping maintain a stable capacity and energy density for the full cells.”
Co-authors are Rice graduate students Quan Anh Nguyen and Botao Farren Song, and Rachel Blaser, a research and development engineer at Ford Motor Co. Biswal is a professor of chemical and biomolecular engineering and materials science and nanoengineering.
The study in the American Chemical Society’s ACS Applied Energy Materials.