One strategy to reduce the CO2 emissions from concrete manufacturing is to sequester atmospheric CO2 into cement-based products.
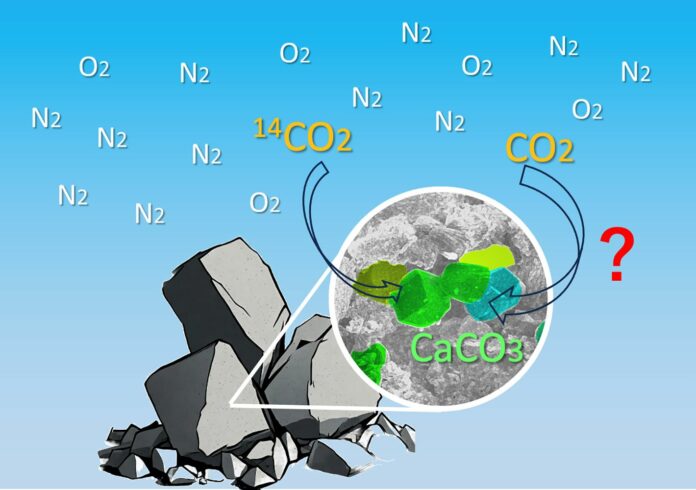
A technique has been devised by researchers at the University of Tokyo and Nagoya University in Japan to verify if the carbon in concrete comes from the raw ingredients or trapped carbon in the air during the reaction that forms the mineral calcium carbonate with the concrete. The team confirmed that direct air carbon capture had taken place by comparing the ratio of specific carbon isotopes in concrete exposed to air and concrete that was not. The industrial sector and nations wishing to reduce carbon emissions may find this strategy helpful.
Carbon dioxide is extracted from the air using physical or chemical methods in direct air capture (DAC). The International Energy Agency’s (IEA) net-zero emissions scenario, which offers a variety of strategies to help the world’s energy industry remove as much carbon dioxide (CO2) as it emits by 2050, includes increasing the usage of DAC technology. In 2022, industry accounted for 25% of CO2 emissions from the global energy system, according to the IEA. After water, cement is the second most used industrial product, although it has a significant negative impact on the environment.
Professor Ippei Maruyama from the Department of Architecture at the University of Tokyo Graduate School of Engineering said, “As much as 800 kilograms of CO2 is emitted per ton of cement during its production, so reducing emissions has become a significant issue in the concrete industry.”
“Concrete has long been known to react with CO2 in the air to form calcium carbonate, an undesirable phenomenon because it induces corrosion of the steel bars inside concrete structures. However, the concrete industry is now considering ways to make effective use of this reaction.”
The process that forms calcium carbonate fixes or traps CO2, eliminating the gas from the environment, although it is troublesome for construction. Additionally, rocks needed to make concrete, such as limestone, naturally contain calcium carbonate.
Maruyama said, “This makes it difficult to distinguish whether or not CO2 identified in concrete has been freshly extracted from the air or comes from rocks. So we developed a method to verify this, which could be used to determine whether the concrete produced can be certified as offsetting CO2 emissions.”
Samples of hydrated cement paste were created for the study to replicate concrete. They pounded the paste sample into powder once it had gotten suitably moist, sealing the powder that was not exposed to the air and leaving the exposed powder uncovered.
They used a method known as accelerator mass spectrometry to analyze the ratio of several carbon isotopes, namely carbon-12, carbon-13, and carbon-14, after seven and twenty-eight days. They did this by dissolving the powder in acid to collect the gas.
Maruyama said, “Fixing carbon dioxide from the air is certified as an act of offsetting CO2 emissions, so it is economically valuable in emissions trading. Digging calcium carbonate for use in concrete is not, so the distinction is crucial, and this research can help support a healthy market.”
“We believe that the carbon neutrality and a circular economy in the construction industry are essential to our future, particularly in Japan where this industry has a role in supporting business continuity and recovery from natural disasters.”
Journal Reference:
- Zhenzhen Wang, Abudushalamu Aili, Masayo Minami and Ippei Maruyama, “Verification method of Direct Air Capture by Cementitious Material Using Carbon Isotopes,” Journal of Advanced Concrete Technology Vol. 21, 934-941: November 22, 2023, DOI: 10.3151/jact.21.934.