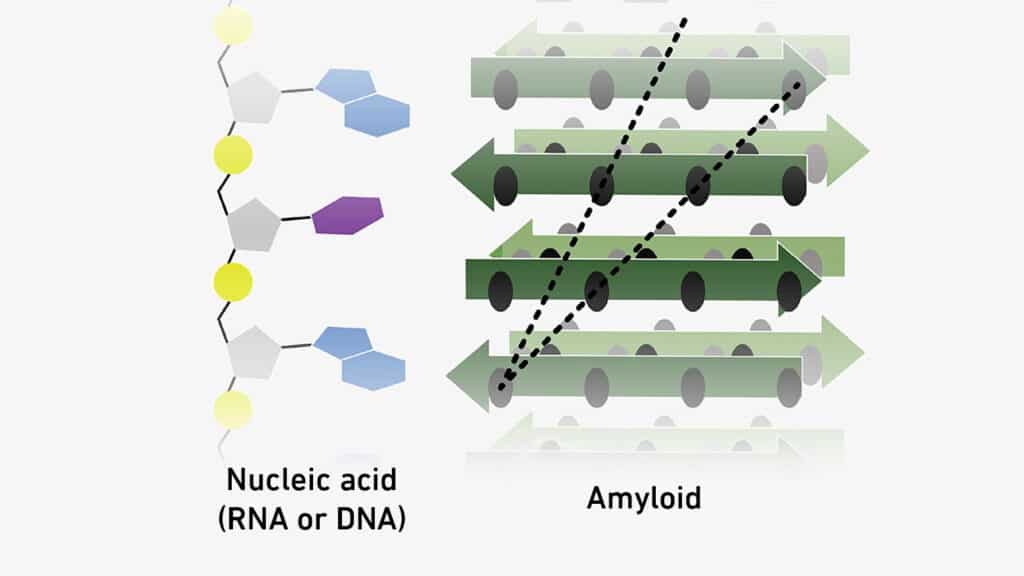
Tiny protein-like clusters called amyloids can stick to genetic material molecules. This might mean that these molecules supported each other as life evolved, possibly even helping to create the genetic code.
Researchers are still figuring out how living things formed from non-living stuff—a big question in science. Even though many ideas have been suggested, there’s no clear answer. It’s not easy because these events happened billions of years ago when Earth was very different than it is now.
Roland Riek, a professor of physical chemistry and associate director of ETH Zurich‘s new interdisciplinary Centre for Origin and Prevalence of Life, said, “Over this vast period of time, evolution has thoroughly obliterated the traces that lead back to the origins of life. Science has no choice but to formulate hypotheses – and to substantiate them as thoroughly as possible with experimental data.”
Riek and his team have been exploring the idea that tiny protein-like groups called amyloids played a vital role in the shift from chemistry to biology. Their initial research showed that amyloids could form quickly in conditions similar to those on early Earth. In the lab, with some volcanic gas and skill, they combined simple amino acids into short peptide chains, naturally forming fibers.
Next, Riek’s team proved that amyloids can make copies of themselves, meeting a crucial requirement to be considered life’s building blocks. In their latest study, they’ve shown for the third time that amyloids can attach to molecules of both RNA and DNA.
The connections happen because of electrical attraction: amyloids are positively charged in certain areas, while genetic material is negatively charged in neutral to acidic surroundings. Additionally, Riek’s team observed that the interactions are influenced by the sequence of RNA and DNA nucleotides in the genetic material. This suggests they could be an early version of the universal genetic code shared by all living things.
Riek said, “Although we see differences in how the RNA and DNA molecules bind with the amyloids, we don’t yet understand what these differences mean. Our model is probably still too simple.”
Riek highlights another crucial aspect of the findings: when genetic material binds to amyloids, both molecules become more stable. This enhanced stability could have been a significant advantage in ancient times.
In the early days, biochemical molecules were spread out thinly in the so-called primordial soup, unlike today’s cells, where these molecules are closely packed. As mentioned in Riek’s recent article, Amyloids can increase the concentration and order of nucleotides in a dilute and disordered system.
Riek emphasizes that while Darwin’s evolution theory focuses on competition, cooperation has also played a significant role. Both molecules benefit from the stabilizing interaction with amyloids because long-lasting molecules accumulate more effectively than unstable ones. It’s possible that cooperation, rather than competition, was crucial in the emergence of life since there was likely an abundance of space and resources in those early times.
His research suggests that working together, tiny clusters called amyloids and genetic material might have been crucial when life began. Amyloids, by sticking to RNA and DNA, not only made things more stable but also made the early environment more organized.
This teamwork, making things steady and collecting long-lasting molecules, goes against the idea that only competition drives evolution. Cooperation could have been essential for life, especially when there was a lot of space and resources.
Journal reference:
- Saroj K. Rout, Riccardo Cadalbert, et al., An Analysis of Nucleotide–Amyloid Interactions Reveals Selective Binding to Codon-Sized RNA. ACS. DOI:10.1021/jacs.3c06287.
