The accumulation of tau protein in the brain is the reason behind cognitive decline in Alzheimer’s and related disorders. The abnormal buildup of tau causes nearby brain tissue to degenerate and die.
Scientists at Washington University School of Medicine in St. Louis discovered that with this buildup of tau deposits, there is also an increase in a type of cholesterol called cholesteryl esters. Reducing cholesterol ester levels aids in preventing behavioral changes and brain damage.
The gene APOE, which activates the immune cells in the brain, is the primary genetic risk factor for Alzheimer’s disease. Such cells can potentially harm brain tissue when stimulated improperly or at the wrong moment. However, APOE performs an additional vital function by transporting lipids, including cholesterol, throughout the circulation. It contributes to atherosclerosis in this way.
Scientists tested mice with a high-risk tau gene predisposing them to accumulate tau in their brains to examine the relationships between APOE, lipids, and brain injury. These mice begin to exhibit neurodegenerative symptoms at six months of age.
By nine and a half months, their brains have suffered significant damage, rendering them incapable of performing routine mouse activities like nest construction. The mice also possessed a second genetic modification: their own APOE genes had been deleted, and they were either wholly replaced or replaced with a human APOE gene variant, such as APOE3, which carries an average risk of Alzheimer’s disease, or APOE4, which has a double or triple risk.
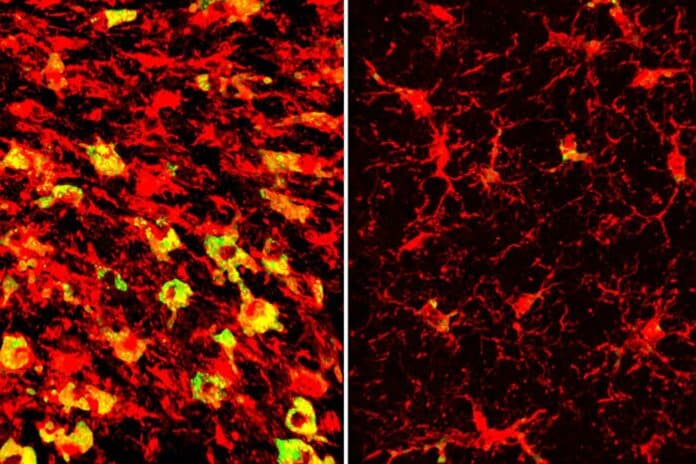
Research has shown that distorted lipid metabolism in the brain is associated with APOE4. The same brain regions injured and atrophied in 9-and-a-half-month-old tau mice carrying APOE4 also oddly accumulated extra lipids.
Over 180 different types of lipids have different levels. One of the most noticeable differences was the abundance of cholesteryl esters called microglia within the immune cells in those regions. The impact of APOE3 was different. Under the direction of Gilbert Di Paolo, Ph.D., experts at Denali Therapeutics collaborated to quantify the brain lipids.
Lipid-filled microglia undergo hyperinflammation and begin secreting neurodegenerative substances. Reducing fats may thus decrease neurodegeneration and inflammation in the brain.
Scientists used an LXR agonist, a medication from an experimental class that decreases cell fat levels, to find out. The drug, GW3965, was given to tau mice with APOE4 beginning at six months of age.
The mice were examined when they were nine and a half months old; their brains should have suffered significant damage by this time. The drug-treated mice maintained a notably larger brain volume than the placebo-treated mice. They also had lower levels of tau, fewer inflammatory cells and less inflammation, less loss of synapses in their brains, and were better at building nests.
Subsequent analysis showed that the LXR agonist functions by activating the Abca1 gene, which aids in removing lipids from cells, including cholesterol. The same benefits of medication therapy were observed when Abca1 levels were raised genetically: decreased lipid buildup, decreased tau, decreased inflammation, and decreased neurodegeneration.
Senior author David M. Holtzman, MD, the Barbara Burton and Reuben M. Morriss III Distinguished Professor of Neurology, said, “What’s exciting is that we see all these effects in an animal model that shares a lot of features with human neurodegenerative diseases. It shows that this kind of approach could have a lot of promise.”
“This work has important therapeutic implications. The compound we used in this study has side effects that make it unsuitable for use in people. But if you could develop a therapy that reduces cholesteryl esters inside brain cells without unacceptable side effects, it would be a promising candidate to test in neurodegenerative diseases.”
Dr. Holtzman explained that while the treatment used in the study isn’t suitable for people due to side effects, finding a way to reduce cholesterol in the brain without causing problems could be a promising treatment for diseases like Alzheimer’s.
LXR agonists have been linked to fatty liver disease because they impact the liver’s lipid metabolism as well. Chemists are working diligently to create LXR agonists devoid of that undesirable side effect. If they succeed, heart disease and brain disease may benefit from the ensuing medications.
Journal Reference:
- Alexandra Litvinchuk, Jung Suh et al. Amelioration of Tau and ApoE4-linked glial lipid accumulation and neurodegeneration with an LXR agonist. Neuron. DOI: 10.1016/j.neuron.2023.10.023