Carbon monoxide is normally considered as toxic for the human body. It is actually produced in our bodies in small amounts that also act as a messenger molecule.
Scientists at Imperial College London along with the Polytechnic University of Valencia have been looking at the other biological roles it can play. They thus developed a new technique that can be used to gauge carbon monoxide levels in the body both effectively and rapidly.
The technique colour-changing strips can potentially show carbon monoxide levels noticeable all around us while maintaining a strategic distance from impedance from steam or smoke. To develop the technique, they initially had to find ways to detect much smaller concentrations, moving from detecting carbon monoxide in parts per million to parts per billion.
The team leader from the Department of Chemistry at Imperial, Dr James Wilton-Ely said, “An additional challenge was to achieve this while avoiding unwanted interactions with the many other substances present. The twin challenges are sensitivity and selectivity, and we think we’ve ticked both boxes.”
Carbon monoxide can be toxic only when it bound with the iron in our blood’s haemoglobin. If this occurs, it keeps its capacity to convey oxygen from the lungs to the rest of the body. In an intriguing turn, this very proclivity for specific metals is the thing that makes the new technique so effective. It utilizes ruthenium with painstakingly composed natural atoms (ligands) appended.
One of these ligands is intended to be able to glow or fluoresce when the light of the precise wavelength is utilized, yet are “turned off” when connected to the ruthenium metal. At the point when carbon monoxide is available, it ties to the ruthenium, separating the ligand. This makes the ligand “switch on” and start to fluoresce under a profoundly infiltrating red light, getting to be noticeable under a magnifying instrument.
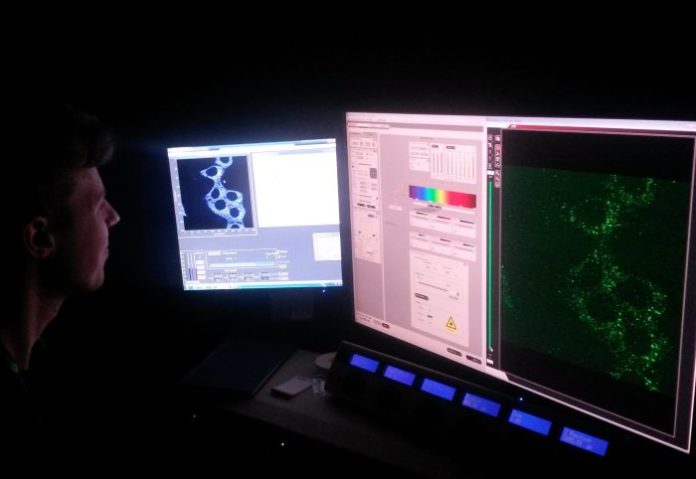
During experiments, scientists used the sample cells of the mice. They found they were quickly able to detect the carbon monoxide produced as a response when inflammation was induced in the mice. Later, they used image analysis to measure the amount of carbon monoxide present from the fluorescence delivered.
Dr Wilton-Ely said, “This technology has great potential for future applications in medical diagnostics, but it also illustrates the different ways we should be thinking about chemicals like carbon monoxide. This offers a really nice insight into the world around us – so many things have multiple roles and reputations, and this adds a richness to the world of science that should be embraced.”
The study ‘Ex Vivo Tracking of Endogenous CO with a Ruthenium(II) Complex’ is published in the Journal of the American Chemical Society.