Countries worldwide are continuously pursuing green and hygienic technology to generate power from available limited resources of nature. Power generation from renewable energy sources has reached equality with conventional forms. However, the portability of energy derived from cleaner sources has always been challenging.
Conventional batteries use elements such as lithium-ion and lead-acid, which are toxic, have a serious risk of explosion, and are expensive and harmful to the environment.
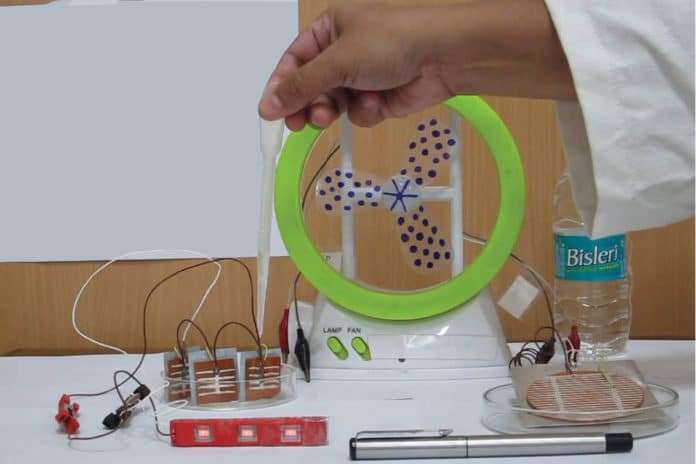
Scientists at Delhi’s National Physical Laboratory (NPL) have found a novel way of producing electricity from water at room temperature without using any power or chemicals. They invented a device called a Hydroelectric Cell that can generate power using only a few drops of water. The device can generate about a quarter ampere current at less than 1V, which is sufficient to power a small plastic fan.
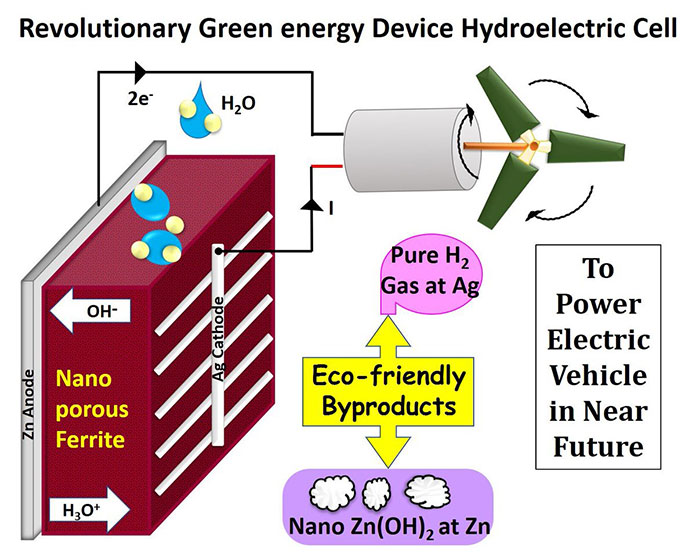
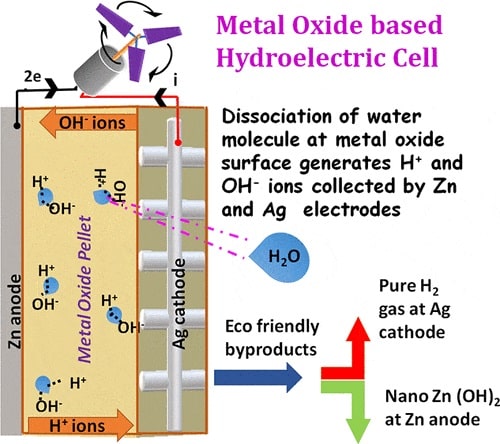
The team led by Dr. Ravinder Kumar Kotnala and his research partner Dr. Jyoti Shah used nonporous magnesium ferrite to split water molecules into hydronium (H3O) and hydroxide (OH) ions spontaneously. Silver and Zinc are used as electrodes to make a cell that produces electricity.
The magnesium ferrite cell of 2-inch diameter generates 82 mA of current and 0.9 V. When four cells of the same dimension are connected in series, the voltage generated can be increased to 3.7 V, enough to power an LED bulb of 1 W. The bulb can be powered for about a week at a stretch, after which zinc hydroxide, the anodic terminal, begins to get trapped in the pores of the magnesium ferrite, thereby diminishing its activity. And it all happens at room temperature.
Since magnesium has a high affinity for hydroxide, it spontaneously splits or dissociates water into hydronium and hydroxide ions. The hydronium ions get trapped inside the nanopores of magnesium ferrite and generate an electric field. The electric field helps in further dissociation of water.
The Hydroelectric Cell has multiple advantages over existing commercial batteries. These cells use commonly available water as a fuel, which replaces the toxic chemicals that threaten the safety of consumers and are harmful to the environment. Moreover, Hydroelectric Cells are expected to be inexpensive once mass-produced, even less than the cost of the solar panel.
The cell releases hydrogen gas and zinc hydroxide, neither of which pollute the environment in the manner that fossil fuels do. Hydroelectric cells are no burden on the environment. It is the world’s first acid and alkali-free cell.
“There are many advantages of the hydroelectric cell compared to electrochemical cells. For instance, they would be portable. Also, in other cells, the anode and cathode get eroded after a period of time. But in hydroelectric cells, Zinc can be reused or recycled and silver extracted out,” says Dr. Kotnala while speaking to the Tech Explorist team.
Now, with the improvement in its design and efficiency, the cell can generate 150 mA current and 0.9 V, nearly twice the earlier one.
Until now, Dr. Kotanala has done an Indian patient and U.S. patent of this invention. Their research was 1st published in The Journal of Physical Chemistry C.