The devices we use daily, like phones and computers, rely on batteries for power. However, in bio-integrated devices—technology designed to interact with our cells at a microscopic level—existing batteries are too large. For example, premature babies in incubators are covered in wires and sticky tape for monitoring vital signs.
A bio-integrated device could eliminate this inconvenience, but a suitable microscopic power source cannot operate and simulate human tissue effectively in such devices.
University of Oxford researchers Yujia Zhang and Linna Zhou from the Hagan Bayley lab have created a miniature battery that can influence the activity of human nerve cells. Drawing inspiration from electric eels, the device utilizes internal ions to generate electricity, mirroring the mechanism found in nature.
The researchers were inspired by a study on electric eels’ energy mechanism, leading them to develop a small-scale hydrogen power source based on the same principles.
Scientists noted, “Maybe we can shrink this down via a droplet technique.”
The envisioned miniature power source was realized using a chain of five nanoliter-sized droplets of a conductive hydrogel—a 3D network of polymer chains containing a significant amount of absorbed water. One strand of human hair is approximately 80,000 to 100,000 nanometers wide to provide a sense of scale.
Each droplet in the chain has a slightly different composition, establishing a salt concentration gradient. Initially, a lipid membrane separates the droplets, preventing ion flow between them. However, when the structure is cooled, it changes, activating its power.
Connecting the droplets at the ends of the chain to electrodes releases and converts their energy into electricity. This electricity allows the hydrogel structure to serve as a power source for external components.
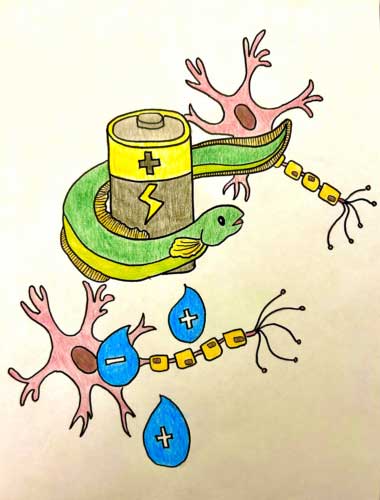
The device also allows living cells to be attached, influencing their activity through the ionic current. When the power source is activated (by cooling the structure), neurons can communicate with each other via calcium signaling.
The initial five droplet units served as a starting point. Combining twenty units in series, the researchers illuminated a two-volt LED light. The team envisions using a droplet printer to create droplet networks of thousands of power units. With this increased power capacity, they aim to sustain bio-integrated devices long-term.
Zhang said, “The major goal of this synthetic tissue project is to be able to interface with real tissues, creating a network between synthetic ones and biological ones. This [project] is only one puzzle piece of the whole puzzle.”
In this project, Zhang’s team successfully stimulated neurons, and they are now extending their work to stimulate heart tissues. Their objective is to create a network comprising both synthetic and real cells. The ultimate goal is to develop a multifunctional interface that can interact with various tissues and organs, not limited to neurons. With a full-body interface, researchers could control communication between different cell types, enabling the study of cell development and tissue regeneration.
Zhang attributes much of their success to the diverse expertise within the team, encompassing engineering, chemistry, and biology.