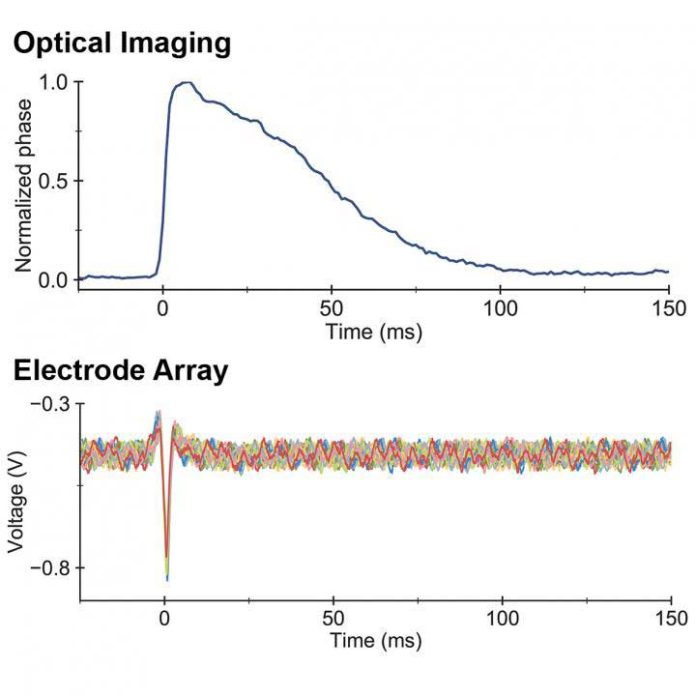
When nerves fire, there’s a change in the electrical potential (trans-membrane voltage) in the cell. Current techniques for monitoring nerve activity are invasive – requiring either electrodes placed near the nerves or fluorescent markers inserted into the cell.
Scientists at Stanford University, Palo Alto, California, have developed a new technology that detects nerve cells fire based on changing shape. According to scientists, the technology can be used for nerve activity in light-accessible body parts, such as the eye.
This non-invasive technology takes benefit of a side-effect of that change in voltage. This means when nerve cells fire, the cell’s membrane temporarily becomes slightly stiffer, leading to a rounding of the cell’s shape. These cell shape changes can be picked up by interferometric (phase) imaging, which senses alterations in the light passing through the cell or being reflected from its surface.
Scientists developed an interferometric microscope outfitted with a fast camera that gathers 50,000 frames for every second. This speed is imperative in light of the fact that the adjustments fit as a fiddle are unpretentious, so there’s almost a signal contrasted with a clamor in the pictures.
With rapid imaging, the specialists can consolidate 50 outlines together in pieces, averaging out the commotion and expanding the signal quality. They likewise structured a novel calculation that would recognize instructive areas (i.e., the parts of the phones that move the most) and support the flag further.
Then, by using a dish of cells engineered to fire like neurons, scientists compared their method to classic, electrode-based measurements of neurons firing. They found that the microscope readings precisely matched the electrical signals sensed by the electrodes.
Through this study, scientists wanted to restore vision loss due to retinal injury or disease. The eventual goal of the project is to use this technology to detect signals passing through the optic nerve or even signals from individual nerve cells in the retina. Direct monitoring of nerves in the eye will help researchers design and test new therapies to restore visual function.
Daniel Palanker, Ph.D., at Stanford, said, “Our task in this joint grant was an establishment of the basic facts—how fast and how much the cells move during action potential—and to devise the best technical strategies for the system to be used in humans. This paper will be a solid reference regarding mechanical effects in cells when they fire.”
Austin Roorda, Ph.D., University of California, said, “Non-invasive, all-optical, neural recording techniques like those pioneered by Dr. Palanker and his team are very exciting because, unlike other methods, these can potentially be used in human eyes. These developments give promise for a day when we can study retinal diseases in human on a cellular scale and evaluate the treatments to cure them.”
Journal Reference
- Ling, T., Boyle, K.C., Goetz, G. et al. Full-field interferometric imaging of propagating action potentials. Light: Science & Applications. 7, 107 (2018). DOI: 10.1038/s41377-018-0107-9