It has been suggested that the Wood-Ljungdahl Pathway functions via nickel-based organometallic intermediates, a distinctive biological mechanism for fixing carbon dioxide and carbon monoxide. But exactly how the pathway proceeds from one step to the next has remained to be determined.
The Wood-Ljungdahl pathway’s previously unknown inner workings have now been uncovered by researchers at the Stanford Synchrotron Radiation Lightsource (SSRL) at the Department of Energy’s SLAC National Accelerator Laboratory, University of Michigan, Northwestern University, and Carnegie Mellon University. The study sheds light on one of the oldest chemical reactions on Earth.
Before this study, scientists knew that the Wood-Ljungdahl pathway generates carbon for organisms using carbon dioxide. It then converts CO2 to carbon monoxide and a methyl group. Later, through some chemistry, magic merges them into a form of carbon that the organism can use.
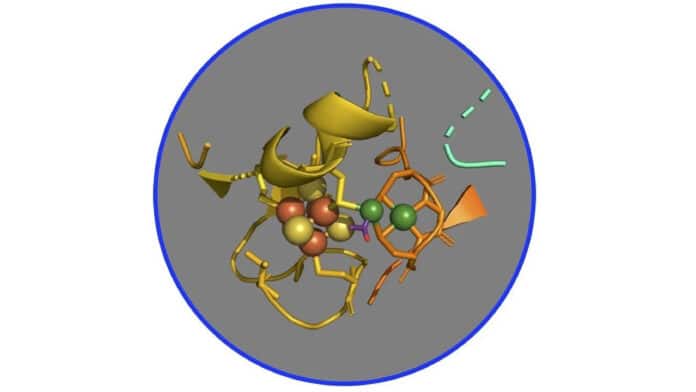
Scientists have hypothesized that the pathway involves several nickel-based organometallic intermediates that link metal to carbon for many years. A complex of two nickel-iron-sulfur proteins known as CO dehydrogenase and acetyl-CoA synthase (CODH/ACS), which are the main enzymes that catalyze the conversion of carbon dioxide into energy and structural carbon for constructing cell walls and proteins, has received particular attention from researchers.
A scarcity of oxygen in the atmosphere, like that of early Earth 4 billion years ago when these proteins and this route first appeared, makes it difficult to confirm this theory. Additionally, the reaction can abruptly stop working because the intermediate molecules are frequently unstable. The existence of additional nickel and iron atoms in the CODH makes it difficult to analyze the ACS, the study’s primary objective.
To overcome these difficulties, the researchers created a more active form of the protein, solely ACS, and did not contain CODH. They also employed X-rays at SSRL to study the metals in the protein and how they function within the enzyme. The group used X-ray spectroscopy, a method that allows scientists to examine the interference of light absorbed by, emitted from, and then reflected by the metals in a complex – in this case, the ACS – to identify altering chemical bonds as reactions occur.
In short, the scientists confirmed their long-standing hypothesis.
Ritimukta Sarangi, senior scientist at SSRL and a corresponding author on the study, said, “What we found is that there is a very intricate bit of organometallic chemistry that’s going on where a single nickel site in the enzyme is doing all the fun stuff.”
“We learned that although the enzyme has a cluster of two nickels bound to four atoms each of iron and sulfur, the reaction always occurs at one specific nickel within the cluster.”
Steve Ragsdale, a professor at the University of Michigan and corresponding author on the study, said, “The carbons, such as carbon monoxide, the methyl group, and the acetyl group, all bind to the nickel closest to the iron and sulfur, and it’s very clear that they don’t bind to any of the other metals.”
“We also noticed that the nickel-containing protein undergoes major changes in its structure at each one of the intermediate states. That’s something that was not part of our original hypothesis. We were thinking about the basic chemistry being nickel-based. But then we see all these other changes in the protein, which was a little bit surprising.”
The research is expected to help understand several natural processes better and could also enhance those processes. It could also help develop methods for mitigating climate change and developing carbon capture to make chemical feedstocks and fuels.
Journal Reference:
- Mehmet Can, Macon J. Abernathy, Seth Wiley, et al. Characterization of Methyl- and Acetyl-Ni Intermediates in Acetyl CoA Synthase Formed during Anaerobic CO2 and CO Fixation. Journal of the American Chemical Society. DOI: 10.1021/jacs.3c01772