Among the collection of greenhouse gases, carbon dioxide (CO2) is the one that has attracted the most attention with regard to global warming. This is likely because CO2 is a by-product of burning fossil fuels, the acknowledged predominant energy source for our nation’s and the world’s economy.
Now, scientists at the RMIT University have made a breakthrough by successfully transforming carbon dioxide into solid coal. They have developed a new technique using liquid metals that can efficiently convert CO2 from a gas into solid particles of carbon.
Scientists noted that this breakthrough could potentially offer other possible ways to remove greenhouse gases from our environment without hazarding.
In recent years, researchers have discovered a handful of solid metal catalysts—compounds that speed up chemical reactions—that can convert CO2 into solid carbon. But these work only above 600°C, and providing that heat requires a lot of energy—and money. The catalysts also gum up quickly, when the carbon they produce builds up, limiting their ability to keep the reactions going.
To overcome this issue, scientists designed a liquid metal catalyst with specific surface properties that made it extremely efficient at conducting electricity while chemically activating the surface. The carbon dioxide is dissolved in a beaker filled with an electrolyte liquid and a small amount of the liquid metal, which is then charged with an electrical current.
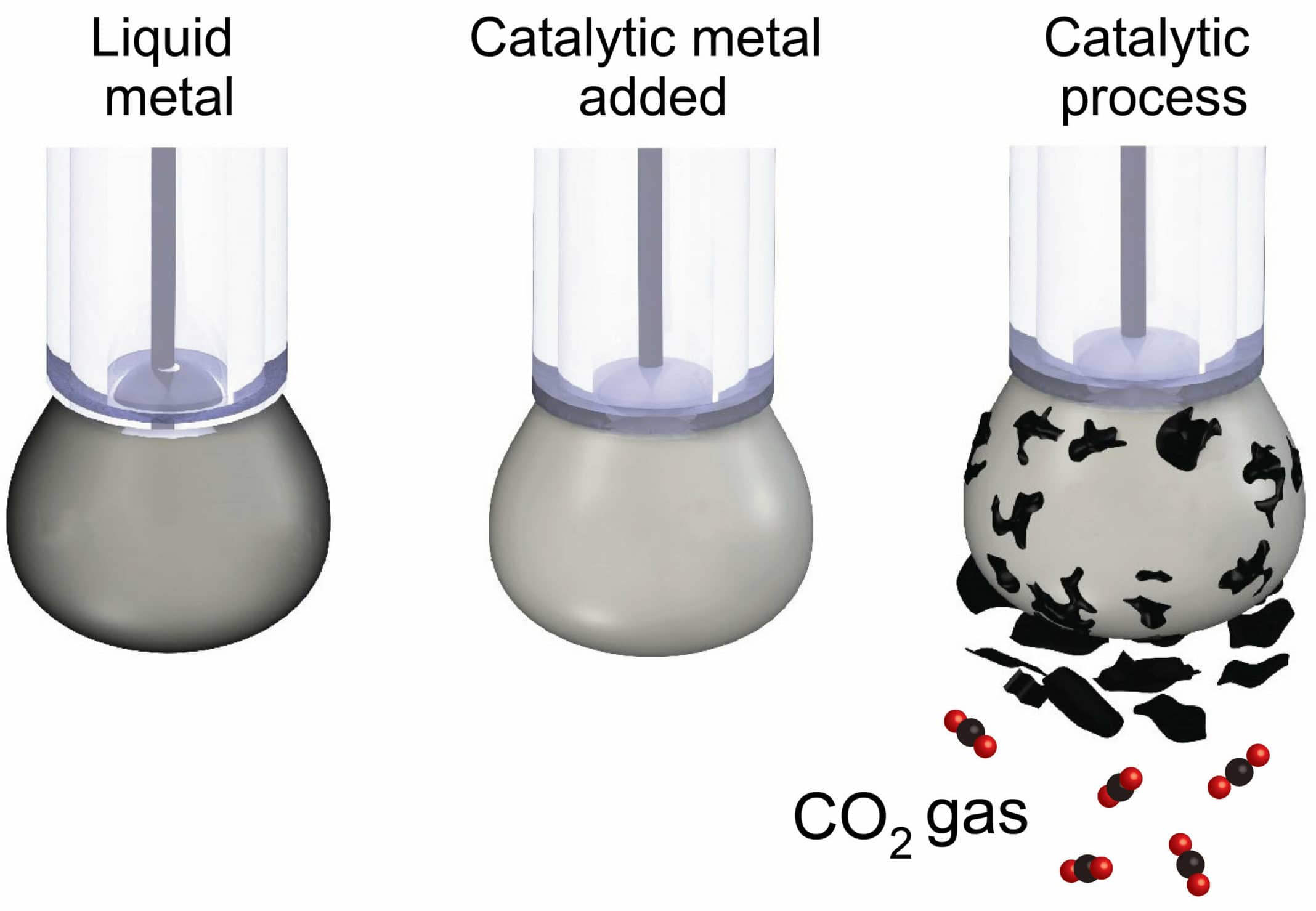
The CO2 slowly converts into solid flakes of carbon, which are naturally detached from the liquid metal surface, allowing the continuous production of carbonaceous solid.
Lead author, Dr. Dorna Esrafilzadeh, a Vice-Chancellor’s Research Fellow in RMIT’s School of Engineering said, “A side benefit of the process is that the carbon can hold an electrical charge, becoming a supercapacitor, so it could potentially be used as a component in future vehicles. The process also produces synthetic fuel as a by-product, which could also have industrial applications.”
RMIT researcher Dr. Torben Daeneke said converting CO2 into a solid could be a more sustainable approach.
“While we can’t literally turn back time, turning carbon dioxide back into coal and burying it back in the ground is a bit like rewinding the emissions clock. To date, CO2 has only been converted into a solid at extremely high temperatures, making it industrially unviable.”
“By using liquid metals as a catalyst, we’ve shown it’s possible to turn the gas back into carbon at room temperature, in a process that’s efficient and scalable.”
“While more research needs to be done, it’s a crucial first step to delivering solid storage of carbon.”
The paper is published in Nature Communications.
The research was conducted at RMIT’s MicroNano Research Facility and the RMIT Microscopy and Microanalysis Facility, with the lead investigator, Honorary RMIT, and ARC Laureate Fellow, Professor Kourosh Kalantar-Zadeh (now UNSW).
The research is supported by the Australian Research Council Centre for Future Low-Energy Electronics Technologies (FLEET) and the ARC Centre of Excellence for Electromaterials Science (ACES).
The collaboration involved researchers from Germany (University of Munster), China (Nanjing University of Aeronautics and Astronautics), the US (North Carolina State University) and Australia (UNSW, University of Wollongong, Monash University, QUT).
