Alzheimer’s disease is an irreversible, progressive brain disease in which amyloid-beta and another protein called tau to build up into tangles and plaques – known collectively as aggregates. This protein aggregate causes brain cells to die and the brain to shrink, resulting in memory loss, personality changes, and difficulty carrying out daily functions.
It is still unknown what kind of biochemical changes inside a cell lead to amyloid-beta aggregation. A new study investigates the possible link between temperature and amyloid-beta aggregation in human cells.
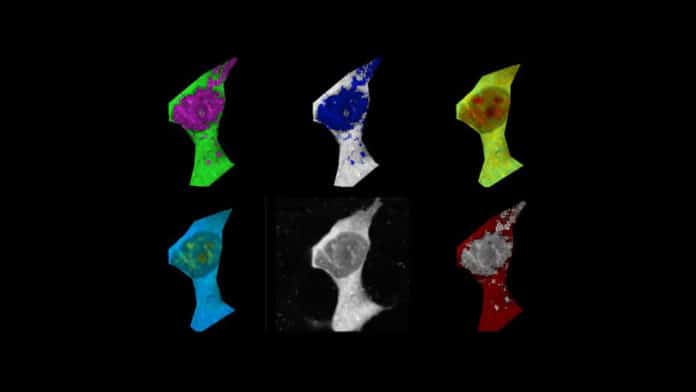
Using small and sensitive sensors, scientists from the University of Cambridge detected the temperature changes inside individual cells. They found that amyloid-beta misfolds and clumps together, causing cells to overheat. The heat released by amyloid-beta aggregation causes other healthy amyloid-beta to be aggregate, causing more and more aggregates to form.
Scientists found that lowering the cell temperature using a drug compound could stop amyloid-beta aggregation. This suggests that the compound could potentially be used to treat Alzheimer’s disease. However, extensive tests and clinical trials would first be required.
Chyi Wei Chung, the study’s first author, said, “The field of studying temperature changes inside a cell is known as intracellular thermogenesis. Thermogenesis has been associated with cellular stress, which may promote further aggregation.”
“We believe that cellular temperatures increase when there’s an imbalance in cells, like when the amyloid-beta concentration is slightly too high, and it starts to accumulate.”
Kaminski Schierle, who led the research, said, “Overheating a cell is like frying an egg – as it heats up, the proteins start to clump together and become non-functional.”
Scientists studied the link between aggregation and temperature using tiny temperature sensors called fluorescent polymeric thermometers (FTPs). They employed amyloid-beta to initiate the aggregation process in human cell lines and a chemical called FCCP as a control because it is known to cause a temperature increase.
As amyloid-beta began to form fibrils and thread-like clumps, the average temperature of the cells began to rise. When compared to cells with no amyloid-beta, the increase in cellular temperature was considerable, found scientists.
Kaminski Schierlesaid, “As the fibrils start elongating, they release energy in the form of heat. Amyloid-beta aggregation requires quite a lot of energy to get going, but once the aggregation process starts, it speeds up and releases more heat, allowing more aggregates to form.”
Chung said, “Once the aggregates have formed, they can exit the cell and be taken up by neighboring cells, infecting healthy amyloid-beta in those cells. No one has shown this link between temperature and aggregation in live cells before.”
Using a drug that inhibits amyloid-beta aggregation, scientists pinpoint the fibrils as the cause of thermogenesis. It had previously been unknown whether protein aggregation or potential damage to mitochondria – the ‘batteries’ that power cells – was responsible for this phenomenon.
Scientists also found that the rise in cellular temperatures could be mitigated by treating them with an aggregation inhibitor, highlighting its potential therapeutic for Alzheimer’s disease.
The laboratory experiments were complemented by computational modeling describing what might happen to amyloid-beta in an intracellular environment and why it might lead to an increase in intracellular temperatures. The researchers hope their work will motivate new studies incorporating different parameters of physiological relevance.
Journal Reference:
- Chyi Wei Chung, Amberley D. Stephens et al. Intracellular Aβ42 Aggregation Leads to Cellular Thermogenesis. DOI: 10.1021/jacs.2c03599