Since the time of Charles Darwin, explaining the stepwise evolution of the eye has been challenging. While numerous evolutionary transitions that led to the vertebrate eye have been explained, some aspects appear to be vertebrate-specific with no obvious metazoan precursor.
One critical difference between vertebrate and invertebrate vision hinges on interphotoreceptor retinoid-binding protein (IRBP, also known as retinol-binding protein, RBP3). This enables cells’ physical separation and specialization in the vertebrate visual cycle by promoting retinoid shuttling between cell types.
Scientists in the University of California San Diego School of Biological Sciences, publishing in the Proceedings of the National Academy of Sciences, have now traced the 500 million-year-old origin of vertebrate IRBP to a bacterial source.
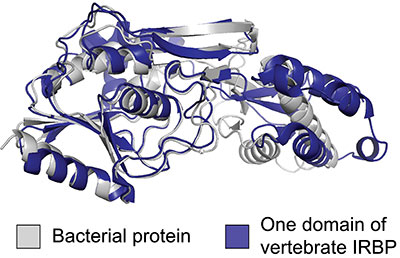
The increasing availability of completely comprehensive genomes has made it possible for their discovery, which was done utilizing phylogenetic reconstruction techniques. The IRBP integration in vertebrate eyes was not the result of conventional vertical gene transfer, in which evolutionary progress is adapted, or “tinkered with,” using accessible genetic material, according to their research of more than 900 genomes from across the Tree of life. Instead, by horizontal gene transfer from non-host bacterial genes, the IRBP was obtained, duplicated, and integrated.
Biological Sciences Associate Professor Matt Daugherty, the paper’s senior author, said, “It’s a massive shift because this is an entirely new piece of genetic material introduced from bacteria. This study shows that a major innovation that distinguishes vertebrate eyes from all the other eyes out there wasn’t done by molecular tinkering but rather a big leap of genetic innovation.”
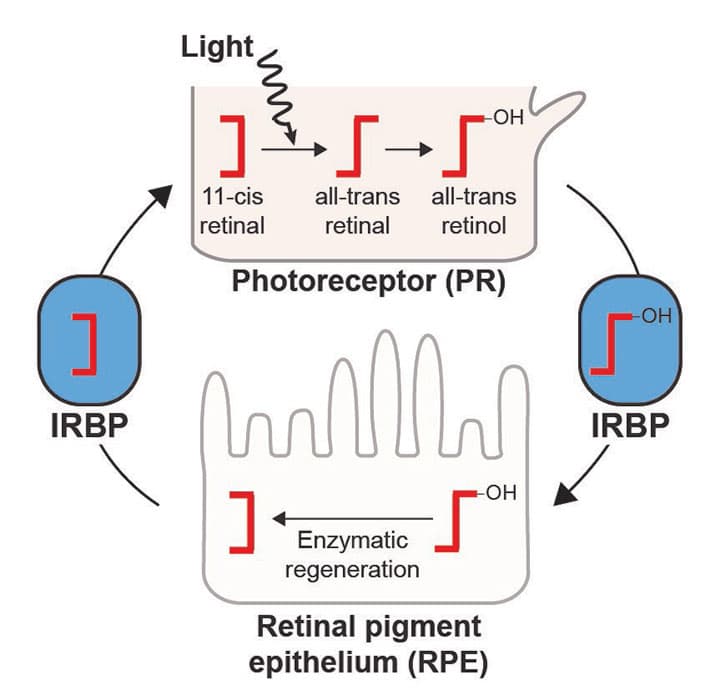
Once the crucial gene that eventually gave rise to IRBP was acquired from bacteria, a novel pathway was made possible in vertebrates, enabling retinoids—molecules in the eye that directly perceive light—to be efficiently recycled for use in future light sensing. Vertebrates’ vision and photoreception—the separation of light perception and retinoid recycling—offer distinctive functions.
Seeing in different wavelengths require enough light around. That’s one of the arguments for why we can see in the dark well.
“We have this enzymatic recycling system that many invertebrates don’t seem to have,” said Daugherty, a researcher in the Department of Molecular Biology. “Eyes are diverse and complicated, and we’ve gone down this path because of this system.”
The researchers predict that as additional genomes from various creatures become available, other crucial processes and systems will also have bacterial roots.
Daugherty said, “This reshapes the way that we think about evolution and the way we think about complex structures that seem like they’ve emerged out of nowhere.”
Journal Reference:
- Chinmay A. Kalluraya, Alexander J. Weitzel et al. Bacterial origin of a key innovation in the evolution of the vertebrate eye. PNAS. DOI: 10.1073/pnas.2214815120
