All known life needs liquid water to function properly. It’s essential in part because water is such a good solvent, readily dissolving and transporting nutrients across a wide range of temperatures.
Water has been a mysterious source in most of the areas. One such mystery includes the exact point where it comes into contact with air.
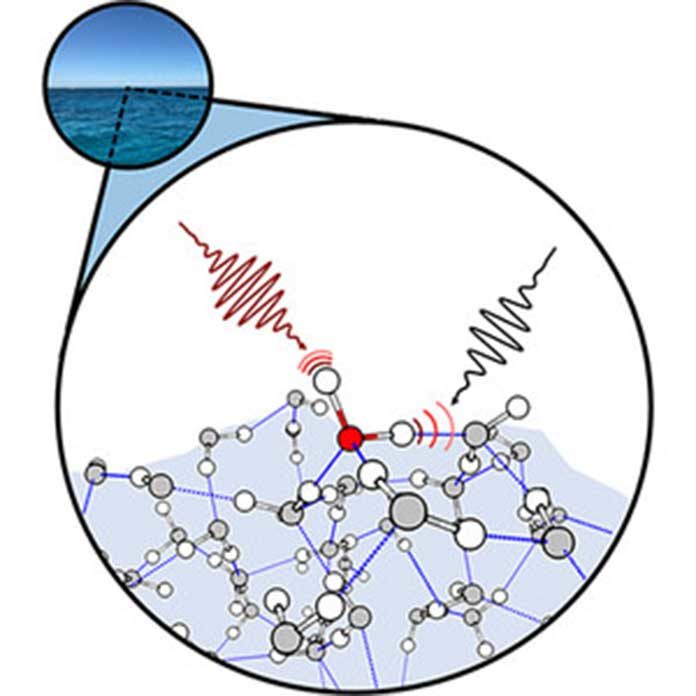
Now, Yale‘s chemistry professor, Mark Johnson, and the University of Washington’s chemistry professor, Anne McCoy, offer a new level of observation and analysis. For the first time ever, they have measured variations in frequency and complexity associated with bonded oxygen and hydrogen (OH) atoms perched on the surface of the water — when one of the OH groups is sticking out of it.
They also measured how these OH groups are coupled together on the surface plane of water.
Johnson said, “Our work is really a fundamental science contribution. Its importance lies in the fact that elementary mechanics and chemical properties of water are important in many fields, and many researchers are involved in simulating this behavior from first principles. We provide a quantitative benchmark upon which to calibrate such simulations.”
Johnson’s work has highlighted a number of the chemical properties of water — often using instruments designed and built at Yale. Among the lab’s many discoveries are innovative uses of electrospray ionization, which was developed by the late Yale Nobel laureate John Fenn, and ways to fast-freeze chemical processes in water to reveal the contorted arrangements of atoms during a reaction.
The study is published in the journal Science.