Using sodium instead of Lithium, scientists at the Imperial College London have created a fast-charging battery prototype. Their new approach could ave a way towards more sustainable batteries.
Scientists used sodium because it is far more abundant than lithium. And as it is easy to extract, thus it requires less mining and is more environmentally friendly. The prototype also uses materials that could replace heavy metals, which are also mining-intensive.
Lead researcher Dr. Florian Glöcklhofer, from the Department of Chemistry and Centre for Processable Electronics at Imperial, said: “Many people have concerns about the widespread use of lithium-ion batteries and their environmental implications, which these new sodium-ion batteries could help address.
“Many people have also experienced the problem of having to charge their devices more often as they get older, and the batteries lose capacity. Our battery prototype did not lose any capacity when tested over 500 charge-discharge cycles, meaning it should not face this problem if developed into a commercial product.”
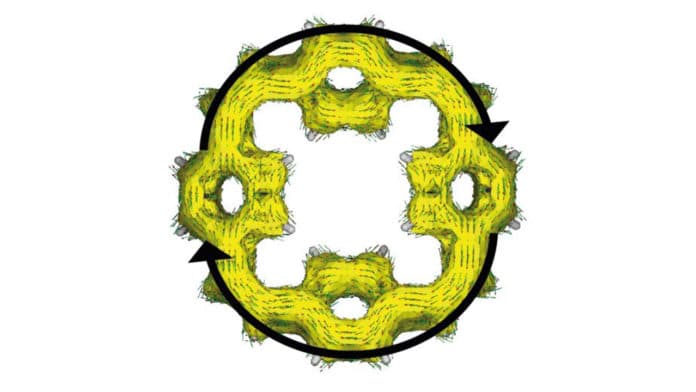
Sodium ions are more significant than lithium ions, and it has been challenging for scientists to discover electrode materials that can store sodium ions. For the new model, scientists utilized organic materials known as ‘macrocyclic atoms’ for the electrodes. These are comprised of just hydrogen and carbon atoms and form ring structures with central holes.
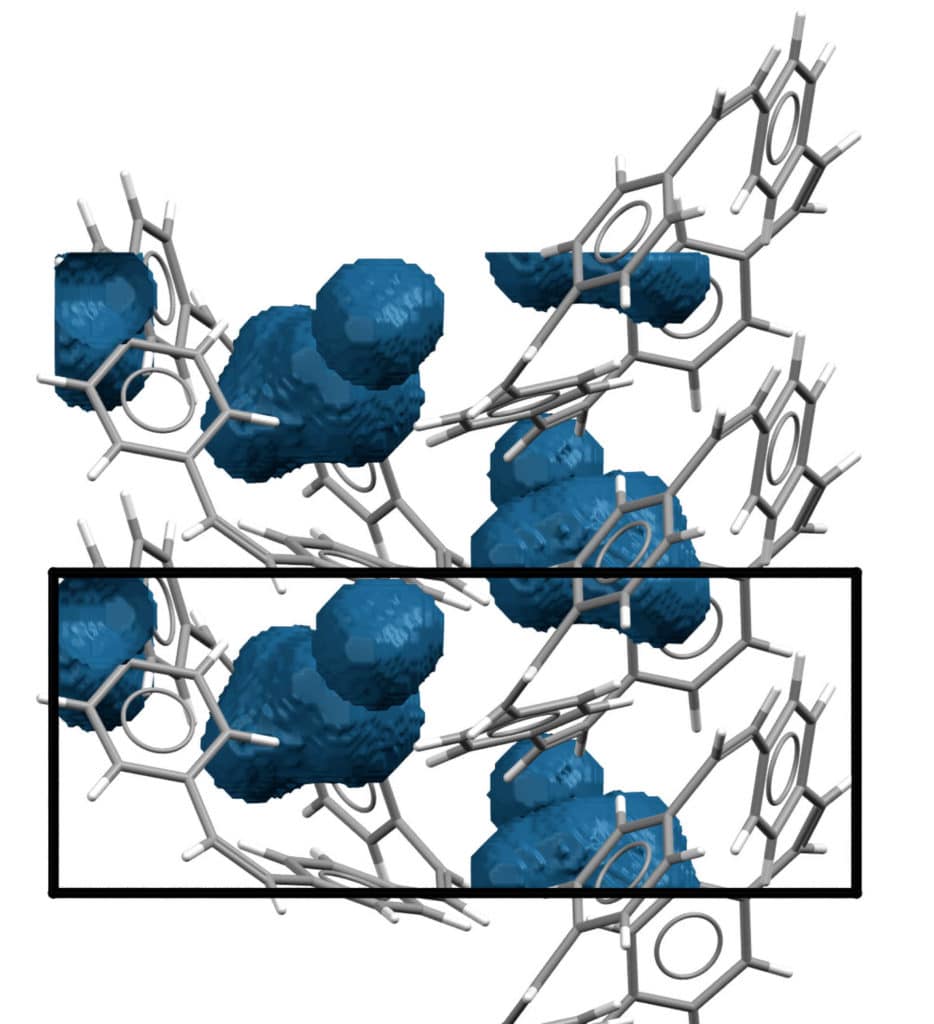
These macrocyclic molecules will always have some space in their center, no matter how densely they are stacked. This space is sufficient to allow sodium ions to be stored without distorting the material.
Due to the ring structure of the macrocyclic molecules, electrons can be distributed around the ring. The more they spread, the less they are less likely to react with elements of the battery. This means the battery remains stable and does not lose any capacity each time it is charged and discharged.
Also, because of the structure of the macrocyclic molecules, two electrons are always taken up together. This increases performance and making charging fast.
While the battery prototype can be charged and discharged quickly and is stable over many cycles of charge and discharge, the overall power capacity is lower than commercial lithium-ion batteries. The team is working to improve the electrode materials and raise their ability, potentially bringing sodium-ion batteries to the market in the future.
Journal Reference:
- Simon Eder et al., Switching between local and global aromaticity in a conjugated macrocycle enables high-performance organic sodium-ion battery anodes. DOI: 10.1002/anie.202003386