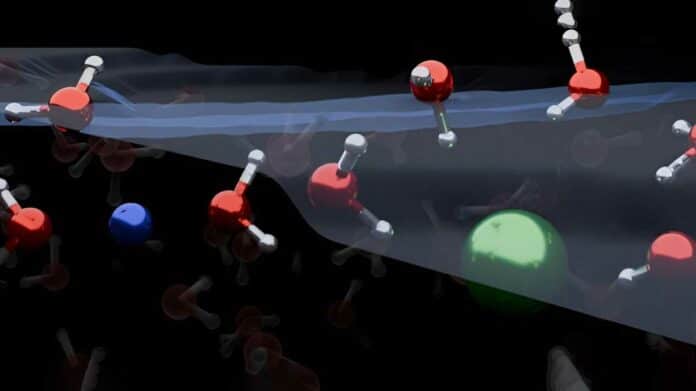
The arrangement of ions at the air/water interface is crucial in various natural processes. Studies suggest that larger ions often exhibit surface activity, meaning they are positioned at the water surface, influencing electric fields that, in turn, shape the structure of water at the interface.
However, the detailed comprehension of the microscopic reactions at these crucial interfaces has been a topic of intense debate.
A new study challenges this view by combining surface-specific heterodyne-detected vibrational sum-frequency generation with neural network-assisted ab initio molecular dynamics simulations. The study by the University of Cambridge and the Max Planck Institute for Polymer Research in Germany suggests that water molecules at the surface of salt water are organized differently than previously thought.
Scientists have demonstrated that the arrangement of ions and water molecules at the surface of electrolyte solutions (most salt-water solutions) fundamentally differs from what was traditionally understood. This discovery could enhance the accuracy of atmospheric chemistry models and find applications in various fields.
The researchers aimed to investigate how the distribution of ions at the air-water interface affects water molecules. Traditionally, this has been done using vibrational sum-frequency generation (VSFG), a laser radiation technique that directly measures molecular vibrations at these interfaces. However, VSFG only measures signal strength, not the signal’s polarity, leading to past difficulties in interpretation.
The team addressed this using a more advanced form of VSFG called heterodyne-detected (HD)-VSFG to study different electrolyte solutions. They also created sophisticated computer models to simulate different scenarios.
Their findings revealed that both positively charged ions (cations) and negatively charged ions (anions) are depleted from the water/air interface. Additionally, these ions orient water molecules in both up- and down orientations, contrary to traditional models predicting a single orientation.
Co-first author Dr. Yair Litman, from the Yusuf Hamied Department of Chemistry, said: “Our work demonstrates that the surface of simple electrolyte solutions has a different ion distribution than previously thought and that the ion-enriched subsurface determines how the interface is organised: at the very top there are a few layers of pure water, then an ion-rich layer, then finally the bulk salt solution.”
Co-first author Dr Kuo-Yang Chiang of the Max Planck Institute said: “This paper shows that combining high-level HD-VSFG with simulations is an invaluable tool that will contribute to the molecular-level understanding of liquid interfaces.”
Professor Mischa Bonn, who heads the Molecular Spectroscopy department of the Max Planck Institute, added: “These types of interfaces occur everywhere on the planet, so studying them not only helps our fundamental understanding but can also lead to better devices and technologies. We are applying these same methods to study solid/liquid interfaces, which could have potential applications in batteries and energy storage.”
Journal Reference:
- Litman, Y., Chiang, KY., Seki, T. et al. Surface stratification determines the interfacial water structure of simple electrolyte solutions. Nat. Chem. (2024). DOI: 10.1038/s41557-023-01416-6