The formation and growth of water-ice layers on surfaces and of low-dimensional ice under confinement are frequent occurrences. Although, this process has kept physicists and chemists puzzled and busy figuring out the details for decades.
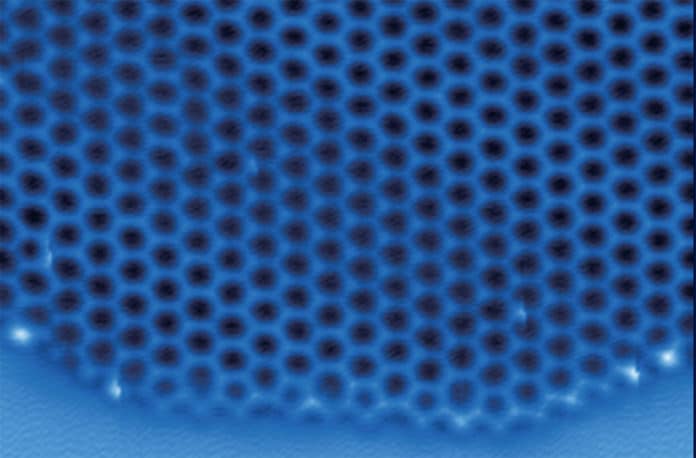
Now, in a new study, researchers depict the first-ever visualization of the atomic structure of two-dimensional ice. The discovery was made by using computer simulations and could offer ways to design materials that make ice removal a more straightforward and less costly process.
Coauthor Chongqin Zhu, a postdoctoral fellow in Francisco’s group who led much of the computational work for the study, said, “Knowing the structure is essential. Low-dimensional water is ubiquitous and plays a critical role in an incredibly broad spectrum of sciences, including materials science, chemistry, biology, and atmospheric science.”
“It also has practical significance. For example, removing ice is critical when it comes to things like wind turbines, which cannot function when they are covered in ice. If we understand the interaction between water and surfaces, then we might be able to develop new materials to make this ice removal easier.”
Since years, scientists are trying to understand the behavior of water, and specifically ice, at the interface of solid surfaces. What they’ve found out about ice’s growth mechanisms and structures in this context encourages them to see how ice behaves in progressively complex scenarios similar to while collaborating with different synthetic compounds and water vapor in the air.
Francisco explained, “We’re interested in the chemistry of ice at the transition with the gas phase, as that’s relevant to the reactions that are happening in our atmosphere.”
In past studies, scientists used computational methods and simulations and showed that ice grows differently depending on whether a surface repels or attracts water and the structure of that surface.
In this work, scientists sought real-world verification of their simulations, reaching out to a team at Peking University to see if they could obtain images of two-dimensional ice.
Then by using super-powerful atomic force microscopy, which uses a mechanical probe to “feel” the material being contemplated, interpreting the feedback into nanoscale-resolution images. Atomic force microscopy is capable of capturing structural information with at least interruption to the material itself, enabling the scientists to distinguish even unstable intermediate structures that emerged during the procedure of ice formation.
Virtually all naturally occurring ice on Earth is known as hexagonal ice for its six-sided structure. This is why snowflakes all have a six-fold symmetry. One plane of hexagonal ice has a similar structure to that of two-dimensional ice and can terminate in two types of edges—”zigzag” or “armchair.” Usually, this plane of natural ice terminates with zigzag edges.
However, when ice grows in two dimensions, the pattern of growth is different. The current work, for the first time, shows that the armchair edges can be stabilized and that their growth follows a novel reaction pathway.
In addition to lending insight into the future design of materials conducive to ice removal, the techniques used in work are also applicable to probe the growth of a large family of two-dimensional materials beyond two-dimensional ices, thus opening a new avenue of visualizing the structure and dynamics of low-dimensional matter.
Chemist Jeffrey Saven, a professor in Penn Arts & Sciences, said, “An experimentalist is talking with theorists about data collected in the lab. The mediocre theorist says, ‘I can’t explain your data.’ The good theorist says, ‘I have a theory that fits your data.’ The great theorist says, ‘That’s interesting, but here is the experiment you should be doing and why.'”
Zhu said, “Looking for features of three-dimensional ice will be the next step and should be very important in looking for applications of this work.”
The paper is presented in the journal Nature.