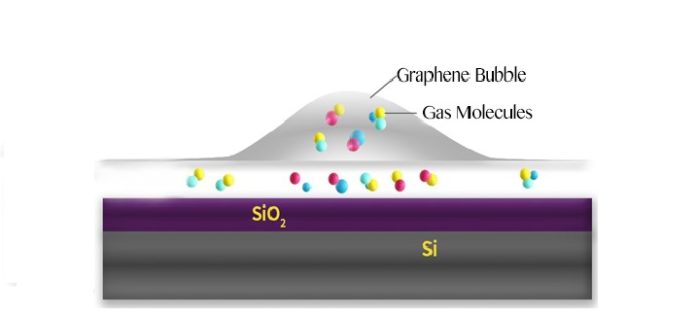
A recent study by the UNIST scientists has identified that graphene bubbles are found to be more chemically reactive than flat graphene. They quantified and controlled the temperature of individual graphene bubbles with a single laser beam for the first time.
Scientists believe that the study could demonstrate the potential of Raman Spectroscopy. Thus, it will offer essential data on the physical properties, and the chemical reactivity of graphene.
The elasticity and flexibility property of graphene allows it create large bubbles. The strain and curvature introduced by the bubbles are known to tune the electronic, chemical, and mechanical properties of this material.
By and large, graphene bubbles are more receptive than level graphene, so they may be more inclined to be embellished with concoction gatherings. Air pockets may fill in as modest, shut reactors, and their bent surface could give a focal point of impact. Seeing how temperature shifts inside air pockets is a vital factor for a few applications.
This study has been led by Distinguished Professor Rodney S. Ruoff (School of Natural Science) in collaboration with Distinguished Professor Feng Ding (School of Materials Science and Engineering) from the Center for Multidimensional Carbon Materials (CMCM) within the Institute for Basic Science (IBS) at UNIST. Their findings have been published in Physical Review Letters on May 3, 2018.
Dr. Yuan Huang, the first author of the study said, “If you think that chemical reactions could be carried out inside the bubble or on the surface of each graphene bubble, then changing the temperature distribution in a bubble will significantly influence reactions taking place.”
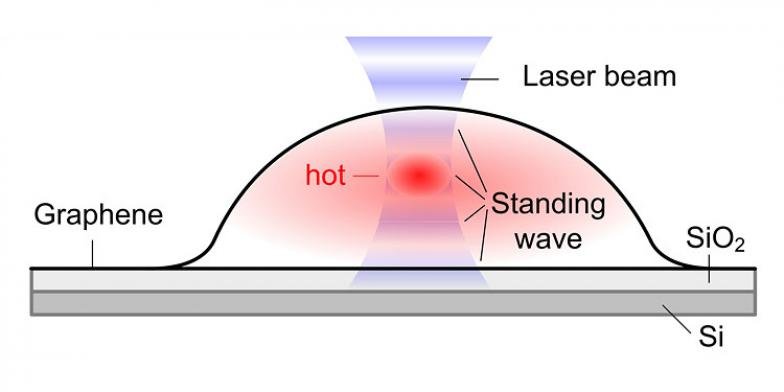
During experiments, bubbles form when molecules are trapped between the graphene sheet and the silica (SiO2/Si) substrate. The hottest spot is marked in red, which corresponds to the highest part of the bubble.
Dr. Xiao Wang and Professor Ding predicted that “the temperature oscillates with the bubble height. Although each bubble is only several micrometers in width and about one micrometer in height, the scientists could detect a variation in temperature, not only between the center and the edges but also at different heights of the bubble.”
“When a graphene bubble is illuminated with a laser beam, incident and reflected rays overlap forming an optical standing wave on the surface.”
Increasing the laser power has the effect of selectively heating specific regions of the bubble, which correspond to the maximum interference of the standing optical wave. Doing this scientists observed changes in temperature within each bubble.
Wolfgang Bacsa, one of the members of the team said, “The results are surprising. The laser beam can efficiently heat the graphene, and we can determine the thermal conductivity in graphene bubbles from its temperature distribution.”
Professor Ruoff, coauthor, and director of CMCM said, “These results confirm the high thermal conductivity of graphene previously measured, demonstrate the excellent adhesion around the perimeter of the graphene bubble, and provide new perspectives on how to heat graphene bubbles on specific locations. The more we know about the physical properties of graphene bubbles, the more we might be able to make use of them in different ways.”