When a molecule interacts with light, its electrons can absorb energy from the electromagnetic field by rapidly rearranging their positions.
This subtle rearrangement makes ready for everything that follows and decides how the reaction proceeds.
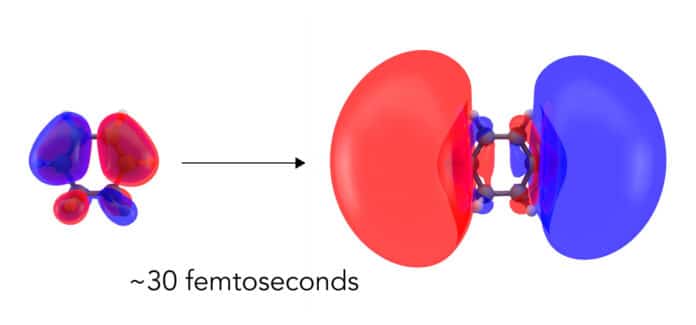
Now, scientists from Brown University, the University of Edinburgh, and the Department of Energy’s SLAC National Accelerator Laboratory have directly observed how the molecule’s electron cloud balloons out before any of the atomic nuclei in the molecule respond.
This is for the first time that scientists have directly imaged this response with X-rays in a process known as molecular movie-making. Their ultimate goal was to observe how both electrons and nuclei act in real-time when chemical bonds form or break.
Peter Weber, a chemistry professor at Brown and senior author of the study, said, “In past molecular movies, we have been able to see how atomic nuclei move during a chemical reaction. But the chemical bonding itself, which is a result of the redistribution of electrons, was invisible. Now the door is open to watching the chemical bonds change during reactions.”
For the study, scientists hit samples of 1,3-cyclohexadiene (CHD) gas with a wavelength of laser light. Doing so excited the molecules into a state that lives for a relatively long period —200 femtoseconds, or millionths of a billionth of a second—so their electronic structure could be probed with LCLS X-ray laser pulses.
Adam Kirrander, a senior lecturer at Edinburgh and senior co-author of the study, said, “X-ray scattering has been used to determine the structure of matter for more than 100 years, but this is the first time the electronic structure of an excited state has been directly observed.”
Non-resonant X-ray scattering technique was used to quantify the arrangement of electrons in the sample. Their measurement unveils: While the signal from the electrons was weak, the researchers were able to capture how the electron cloud deformed into a more giant unambiguously, more diffuse cloud corresponding to an excited electronic state.
Haiwang Yong, a Ph.D. student at Brown University and lead author of the report, said, “In a chemical reaction, the atomic nuclei move, and it’s difficult to disentangle that signal from the other parts that belong to chemical bonds forming or breaking. In this study, the change in the positions of atomic nuclei is comparatively small on that timescale, so we were able to see the motions of electrons right after the molecule absorbs light.”
SLAC senior staff scientist Michael Minitti added, “We’re imaging these electrons as they move and shift around. This paves the way to watching electron motions in and around bond breaking and bond formation directly and in real-time; in that sense, it’s similar to photography.”
Journal Reference:
- Haiwang Yong, Observation of the molecular response to light upon photoexcitation. DOI: 10.1038/s41467-020-15680-4