Medium- and high-entropy alloys (M/HEAs) are special materials that combine elements in nearly equal amounts. They’re like a new way of designing materials in fields like metallurgy and catalysis. It is believed that changing how the atoms are arranged in these alloys is crucial, but figuring out this arrangement in three dimensions is difficult. The usual methods either mix up the chemical makeup or show unclear images.
Scientists from UCLA have provided an unprecedented view of the structure and characteristics of medium- and high-entropy alloys. They used an advanced imaging technique called atomic electron tomography to map the three-dimensional atomic coordinates of M/HEAs. This study represents the first time their 3D nuclear order has been directly observed.
Medium-entropy alloys mix three or four metals in nearly equal amounts, while high-entropy alloys combine five or more similarly. This is different from regular alloys, where one metal is the main one, and others are in smaller amounts. For instance, stainless steel is mostly iron.
Now, think about a blacksmith making a sword. Surprisingly, small defects in the metal make it stronger. When the blacksmith heats a soft metal bar until it glows and then cools it quickly, these defects build up and turn it into a formidable sword. The scientists are studying how this idea applies to alloys with multiple metals.
Scientists focused on a type of structural defect called a twin boundary. This defect is believed to be a critical factor in medium- and high-entropy alloys’ unique combination of toughness and flexibility.
Twinning occurs when strain causes part of a crystal matrix to bend while the surrounding atoms stay in place, creating mirror images on either side of the boundary.
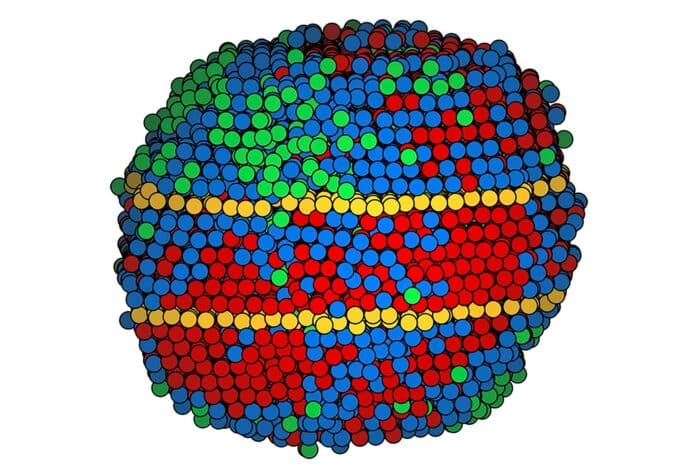
The scientists made tiny nanoparticles using various metals. Six nanoparticles were medium-entropy alloys with nickel, palladium, and platinum. Four were high-entropy alloys with cobalt, nickel, ruthenium, rhodium, palladium, silver, iridium, and platinum.
Creating these alloys is like a super-fast version of a blacksmith’s job. They melted the metal at extremely high temperatures for a fraction of a second, then cooled it down quickly. This rapid process induced twin boundaries in six of the ten nanoparticles, with four having a pair of twins.
The scientists used a special imaging method they created called atomic electron tomography to find these defects. They used electrons because the details at the atomic level are much smaller than the wavelengths of visible light. This method involves capturing multiple images as the sample is rotated, allowing the data to be mapped in 3D.
However, adapting atomic electron tomography to map the intricate mixtures of metals was a challenging process that required careful adjustments.
Miao said, “Our goal is to find the truth in nature, and our measurements must be as accurate as possible. We worked slowly, pushing the limit to make each step of the process as perfect as possible, then moved on to the next step.”
The scientists carefully mapped each atom in the medium-entropy alloy nanoparticles. However, some metals in the high-entropy alloy were too similar in size for electron microscopy to distinguish between them. As a result, the map grouped the atoms into three categories.
They discovered that the more the atoms of different elements (or different categories of elements) were mixed, the more likely the structure of the alloy would change in a way that makes it both rigid and flexible. This finding could be helpful in designing medium- and high-entropy alloys with increased durability. It might also reveal new properties not currently seen in materials like steel, opening up possibilities for engineering alloys with unique characteristics.
Co-author Peter Ercius, a staff scientist at Lawrence Berkeley National Laboratory’s Molecular Foundry, said, “The problem with studying defective materials is that you have to look at each defect separately to know how it affects the surrounding atoms. Atomic electron tomography is the only technique with the resolution to do that. It’s just amazing that we can see jumbled atomic arrangements at this scale inside such small objects.”
Scientists are working on a novel imaging technique that merges atomic electron microscopy with a method for identifying a sample’s composition based on emitted photons. This innovation aims to differentiate between metals with atoms of similar size, overcoming previous challenges.
Additionally, scientists are exploring methods to analyze large quantities of medium- and high-entropy alloys, seeking to unravel the fundamental connections between their structures and properties. These advancements could significantly enhance our understanding of these alloys and open doors to new possibilities in material science.
Journal Reference:
- Moniri, S., Yang, Y., Ding, J. et al. Three-dimensional atomic structure and local chemical order of medium- and high-entropy nanoalloys. Nature 624, 564–569 (2023). DOI: 10.1038/s41586-023-06785-z