Polyester is the second most commonly used textile in the world. It is a major environmental issue due to its inability to be recycled. The fabric, which is made up of a blend of plastic and cotton, has been difficult for the industry to separate and recycle. Researchers from the University of Copenhagen have discovered a surprisingly simple solution to this pressing issue that could revolutionize textile sustainability.
Polyethylene terephthalate (PET) is one of the most commonly used plastics, produced at a rate of 70 million tons per year. One-third of global PET production produces polyester and other synthetic textiles. The widespread use of nonbiodegradable polyethylene terephthalate (PET) materials has created a significant waste management challenge. PET plastic can be recycled. These are harder to recycle because cotton has labile glycosidic bonds.
Recycling polyester poses a significant challenge. There are significant challenges in recycling polyester, particularly separating the plastic and cotton fibers from the blend fabric without losing either of them. Conventional recycling procedures sometimes prioritize conserving the plastic component, resulting in cotton fiber loss. These processes are expensive, difficult and produce metal waste due to the use of metal catalysts, which can be cytotoxic and contaminate the process.
A group of young scientists has made a fantastic discovery in revealing a surprisingly simple solution to this serious problem, potentially transforming the sustainability of the textile industry.

Yang from the University of Copenhagen’s Department of Chemistry, the lead author of the scientific research article, said, “The textile industry urgently requires a better solution to handle blended fabrics like polyester/cotton. Currently, very few practical methods can recycle cotton and plastic. It’s typically an either-or scenario. However, with our newly discovered technique, we can depolymerize polyester into its monomers while recovering cotton on a scale of hundreds of grams, using an incredibly straightforward and environmentally friendly approach. This traceless catalytic methodology could be the game-changer.”
The new recycling method based on hartshorn salt (ammonium bicarbonate) works on PET plastic alone and cotton blend materials. Heat, a non-toxic solvent, and an everyday household component are all required for the new process.
Shriaya Sharma, a doctoral student of the Jiwoong Lee group at the Department of Chemistry and study co-author. Said, “If we throw dirty plastic waste in a container, we still get good quality cotton and plastic monomer out. This can even be a plastic bottle with juice residue still in it. We just put it in and begin the reaction. It still works.”
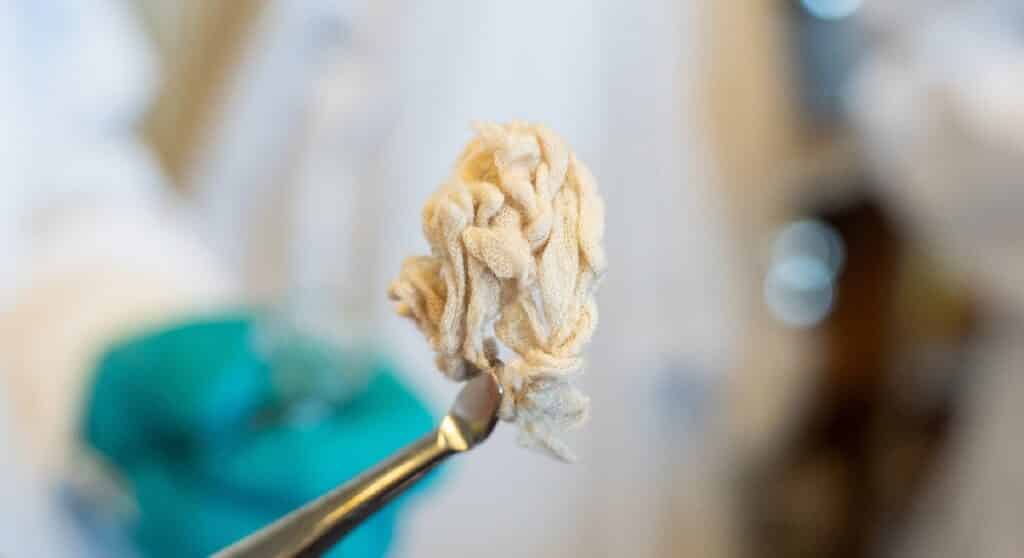
He explains, “For example, we can take a polyester dress, cut it into small pieces and place it in a container. Then, add a bit of mild solvent and hartshorn salt, which many people know as a leavening agent in baked goods. We then heat it all to 160 degrees Celsius and leave it for 24 hours. The result is a liquid in which the plastic and cotton fibers settle into distinct layers. It’s a simple and cost-effective process.”
The hartshorn salt, also known as ammonium bicarbonate, is broken down into ammonia, CO2, and water. The combination of ammonia and CO2 triggers a selective depolymerization reaction that breaks down polyester while preserving cotton fibers. Ammonia is toxic in isolation, but when combined with carbon dioxide (CO2), it becomes safe and environmentally friendly. Cotton fibers remain intact and in excellent condition due to the moderate nature of the chemicals used.
The same research team has shown that CO2 can act as a catalyst to break down other materials, such as nylon, without leaving any residue. They investigated using hartshorn salt after making this discovery. However, the researchers were pleasantly surprised when their simple recipe produced positive outcomes.
Carlo Di Bernardo, doctoral student, and study co-author, said, “We were initially excited to see it work so well on the PET bottles alone. Then, when we discovered that it worked on polyester fabric as well, we were just ecstatic. It was indescribable. That it was so simple to perform was nearly too good to be true.”
The researchers are currently in contact with corporations to test the technology on a larger scale. They hope to commercialize this technology, which has enormous potential because keeping this knowledge within the university gates would be a huge waste.
Journal Reference:
- Yang Yang, Shriaya Sharma, et al. Catalytic Fabric Recycling: Glycolysis of Blended PET with Carbon Dioxide and Ammonia. ACS Sustainable Chemistry & Engineering. DOI: 10.1021/acssuschemeng.3c03114
